Glucan
A glucan is a polysaccharide derived from D-glucose,[1] linked by glycosidic bonds. Glucans are noted in two forms: alpha glucans and beta glucans. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.
Types
The following are glucans (The α- and β- and numbers clarify the type of O-glycosidic bond and the specific carbons involved):[2]
Alpha
- dextran, α-1,6-glucan with α-1,3-branches
- floridean starch, α-1,4- and α-1,6-glucan
- glycogen, α-1,4- and α-1,6-glucan
- pullulan, α-1,4- and α-1,6-glucan
- starch, a mixture of amylose and amylopectin, both α-1,4- and α-1,6-glucans
Beta
- cellulose, β-1,4-glucan
- chrysolaminarin, β-1,3-glucan
- curdlan, β-1,3-glucan
- laminarin, β-1,3- and β-1,6-glucan
- lentinan, a strictly purified β-1,6:β-1,3-glucan from Lentinus edodes
- lichenin, β-1,3- and β-1,4-glucan
- oat beta-glucan, β-1,3- and β-1,4-glucan
- pleuran, β-1,3- and β-1,6-glucan isolated from Pleurotus ostreatus
- zymosan, β-1,3-glucan
Properties
Properties of glucans include resistance to oral acids/enzyme and water insolubility. Glucans extracted from grains tend to be both soluble and insoluble.
Structure
Glucans are polysaccharides derived from glucose monomers. The monomers are linked by glycosidic bonds. Four types of glucose-based polysaccharides are possible: 1,6- (starch), 1,4- (cellulose), 1,3- (laminarin), and 1,2-bonded glucans.
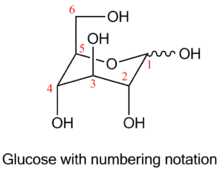
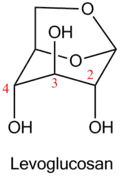
The first representatives of main chain unhydrolysable linear polymers made up of levoglucosan units were synthesized in 1985 by anionic polymerization of 2,3-epoxy derivatives of levoglucosan (1,6;2,3-dianhydro-4-O-alkyl-β-D-mannopyranoses).[3]
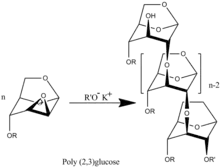
A wide range of unique monomers with different radical R can be synthesized.[4] There were synthesized polymers with R= -CH3,[3] -CH2CHCH2,[5] and -CH2C6H5.[6] Investigation of the polymerization kinetics of those derivatives, molecular weight and molecular-weight distribution showed that the polymerization has the features of a living polymerization system. The process takes place without termination and transfer of the polymer chain with a degree of polymerization equal to the mole ratio of the monomer to the initiator.[7][8] Accordingly, the upper value molecular weight polymer determines only degree of purification system what determine the presence in the system uncontrollable amount of terminators of polymer chains.
Poly(2-3)-D-glucose was synthesized proceeds by transformation of benzyl (R= -CH2C6H5) functionalized polymer.[6]
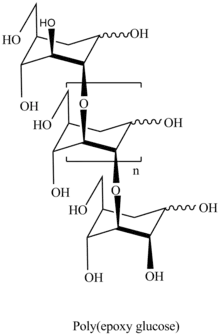
Polymerization of 3,4-epoxy levoglucosan (1,6;3,4-dianhydro-2-O-alkyl-β-D-galactopyranose) results in formation 3,4-bounded levoglucosan polymer.[9]

The presence of 1,6-anhydro structure in every unit of polymer chains allows researchers to apply all spectra of well developed methods of carbohydrate chemistry with formation of highly intriguing biological application polymers. The polymers are the only known regular polyethers built up of carbohydrate units in main polymer chain.[10][11]
Functions
Glucans serve a diverse set of functions. Within the cell, certain glucans store energy, fortify cellular structure, behave in recognition, and enhance virulence in pathogenic organisms.[12]
Glycogen and starch are notable glucans responsible for storing energy for the cell. Receptor molecules of the immune system, such as the Complement receptor 3, or CR3, and CD5 receptor, recognize and bind to beta-glucans on invading cell surfaces.[13]
See also
References
- Glucans at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Synytsya A, Novak M. Structural analysis of glucans. Ann Transl Med. 2014 Feb; 2(2):17. doi:10.3978/j.issn.2305-5839.2014.02.07.
- Berman, E.L., Gorkovenko A.A., Zubov, V.P., and Ponomarenko, V.A., "Regio and Stereospecific Synthesis of Polyglucose with Novel Type Bond" Soviet J.Bioorg. Chem. 11 (1985), 1125-1129
- Carlson, LJ (November 1965). "Preparation of 2- and 4-Substituted D-Glucose Derivatives from 1,6-Anhydro-β-D- glucopyranose". The Journal of Organic Chemistry. 30 (11): 3953–3955. doi:10.1021/jo01022a517.
- Gorkovenko, A.A., Berman, E.L., and Ponomarenko, V.A. Polymerization of 1, 6;2,3 dianhydro 4 O allyl β D manno¬pyranose Vysocomol. Soed., Ser. B, 1987, 29, 134 137
- Gorkovenko, A.A., Berman, E.L., and Ponomarenko, V.A. "A New Polymer of Glucose. Poly(2 3) D glucose" Soviet J. Bioorg. Chem., 1987, 13, 218 222
- Berman, E.L., Gorkovenko, A.A., Rogozhkina, E.D., Izumnikov, A.A., and Ponomarenko, V.A. “Kinetics and Mechanism of Epoxy Ring-Opening Polymerization of 1,6;2,3-Dianhydro-4-O-alkyl-b-D-mannopyranoses” Polymer Sci. USSR, 1988, 413-418
- Berman E.L. Gorkovenko, A.A., Rogozhkina, E.D., Izyumnikov, A.L., and Ponomarenko, V.A. "Synthesis of Chiral Derivatives of Poly(Ethylene Oxide)" Bull. Acad. Sci. USSR, Div. Chem. Sci., 1988, 705 707
- Gorkovenko A. A., Berman, E.L., and Ponomarenko, V.A., Poly(3 4) 2 O methyl 1,6 anhydro b D glucopyranose. The First Example of (3 4) linked Polymer Carbohydrates" Soviet J. Bioorg. Chem. 12 (1986), 514-520
- Berman E.L., Gorkovenko, A.A., and Ponomarenko, V.A. "Structure and Polymerizability of 1,6;2,3 and 1,6;3,4¬ Dianhydrohexapyranoses" Polymer Sci. USSR, 1988, 30, 497¬-502
- Berman, E.L., “New Glucose Polymers” in “Levoglucosenone and Levoglucosanes: Symposium: 204th National meeting”, Zbigniew J. Witczak (editor), American Chemical Society. Division of Carbohydrate Chemistry, 189-214. Publisher: A T L Press, Scientific Publishers ISBN 978-1-882360-13-0 ISBN 1882360133
- Ruiz-Herrera, José; Ortiz-Castellanos, Lucila (2019-12-01). "Cell wall glucans of fungi. A review". The Cell Surface. 5: 100022. doi:10.1016/j.tcsw.2019.100022. ISSN 2468-2330. PMC 7389562. PMID 32743138. S2CID 108720495.
- Goodridge, Helen; Wolf, Andrea; Underhill, David (29 June 2009). "β-glucan recognition by the innate immune system". Immunological Reviews. 230 (1): 38–50. doi:10.1111/j.1600-065X.2009.00793.x. eISSN 1600-065X. ISSN 0105-2896. PMC 6618291. PMID 19594628.