Crystallographic image processing
Crystallographic image processing (CIP) is traditionally understood as being a set of key steps in the determination of the atomic structure of crystalline matter from high-resolution electron microscopy (HREM) images obtained in a transmission electron microscope (TEM) that is run in the parallel illumination mode. The term was created in the research group of Sven Hovmöller at Stockholm University during the early 1980s and became rapidly a label for the "3D crystal structure from 2D transmission/projection images" approach. Since the late 1990s, analogous and complementary image processing techniques that are directed towards the achieving of goals with are either complementary or entirely beyond the scope of the original inception of CIP have been developed independently by members of the computational symmetry/geometry, scanning transmission electron microscopy, scanning probe microscopy communities, and applied crystallography communities.
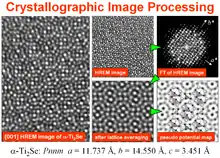
HREM image contrasts and crystal potential reconstruction methods
Many beam HREM images of extremely thin samples are only directly interpretable in terms of a projected crystal structure if they have been recorded under special conditions, i.e. the so-called Scherzer defocus. In that case the positions of the atom columns appear as black blobs in the image (when the spherical aberration coefficient of the objective lens is positive - as always the case for uncorrected TEMs). Difficulties for interpretation of HREM images arise for other defocus values because the transfer properties of the objective lens alter the image contrast as function of the defocus. Hence atom columns which appear at one defocus value as dark blobs can turn into white blobs at a different defocus and vice versa. In addition to the objective lens defocus (which can easily be changed by the TEM operator), the thickness of the crystal under investigation has also a significant influence on the image contrast. These two factors often mix and yield HREM images which cannot be straightforwardly interpreted as a projected structure. If the structure is unknown, so that image simulation techniques cannot be applied beforehand, image interpretation is even more complicated. Nowadays two approaches are available to overcome this problem: one method is the exit-wave function reconstruction method, which requires several HREM images from the same area at different defocus and the other method is crystallographic image processing (CIP) which processes only a single HREM image. Exit-wave function reconstruction [2][3] provides an amplitude and phase image of the (effective) projected crystal potential over the whole field of view. The thereby reconstructed crystal potential is corrected for aberration and delocalisation and also not affected by possible transfer gaps since several images with different defocus are processed. CIP on the other side considers only one image and applies corrections on the averaged image amplitudes and phases. The result of the latter is a pseudo-potential map of one projected unit cell. The result can be further improved by crystal tilt compensation and search for the most likely projected symmetry. In conclusion one can say that the exit-wave function reconstruction method has most advantages for determining the (aperiodic) atomic structure of defects and small clusters and CIP is the method of choice if the periodic structure is in focus of the investigation or when defocus series of HREM images cannot be obtained, e.g. due to beam damage of the sample. However, a recent study on the catalyst related material Cs0.5[Nb2.5W2.5O14] shows the advantages when both methods are linked in one study.[4]
Brief history of crystallographic image processing
Aaron Klug suggested in 1979 that a technique that was originally developed for structure determination of membrane protein structures can also be used for structure determination of inorganic crystals.[5][6] This idea was picked up by the research group of Sven Hovmöller which proved that the metal framework partial structure of the K8−xNb16−xW12+xO80 heavy-metal oxide could be determined from single HREM images recorded at Scherzer defocus.[7] (Scherzer defocus ensures within the weak-phase object approximation a maximal contribution to the image of elastically scattered electrons that were scattered just once while contributions of doubly elastically scattered electrons to the image are optimally suppressed.)
In later years the methods became more sophisticated so that also non-Scherzer images could be processed.[8] One of the most impressive applications at that time was the determination of the complete structure of the complex compound Ti11Se4, which has been inaccessible by X-ray crystallography.[9] Since CIP on single HREM images works only smoothly for layer-structures with at least one short (3 to 5 Å) crystal axis, the method was extended to work also with data from different crystal orientations (= atomic resolution electron tomography). This approach was used in 1990 to reconstruct the 3D structure of the mineral staurolite HFe2Al9Si4O4 [10][11] and more recently to determine the structures of the huge quasicrystal approximant phase ν-AlCrFe [12] and the structures of the complex zeolites TNU-9 [13] and IM-5.[14] As mentioned below in the section on crystallographic processing of images that were recorded from 2D periodic arrays with other types of microscopes, the CIP techniques were taken up since 2009 by members of the scanning transmission electron microscopy, scanning probe microscopy and applied crystallography communities.
Contemporary robotics and computer vision researchers also deal with the topic of "computational symmetry",[15][16][17][18][19] but have so far failed to utilize the spatial distribution of site symmetries that result from crystallographic [20] origin conventions. In addition, a well known statistician noted in his comments on "Symmetry as a continuous feature" [21] that symmetry groups possess inclusion relations (are not disjoint in other words) so that conclusions about which symmetry is most likely present in an image need to be based on "geometric inferences".[22] Such inferences are deeply rooted in information theory, where one is not trying to model empirical data, but extracts and models the information content of the data.[23][24] The key difference between geometric inference and all kinds of traditional statistical inferences is that the former merely states the co-existence of a set of definitive (and exact geometrical) constraints and noise, whereby noise is nothing else but an unknown characteristic of the measurement device and data processing operations. From this follows that "in comparing two" (or more) "geometric models we must take into account the fact that the noise is identical (but unknown) and has the same characteristic for both" (all) "models".[25] Because many of these approaches use linear approximations, the level of random noise needs to be low to moderate, or in other words, the measuring devices must be very well corrected for all kinds of known systematic errors.
These kinds of ideas have, however, only been taken up by a tiny minority of researchers within the computational symmetry [26] and scanning probe microscopy / applied crystallography [27][28] communities. It is fair to say that the members of computational symmetry community are doing crystallographic image processing under a different name and without utilization of its full mathematical framework (e.g. ignorance to the proper choice of the origin of a unit cell and preference for direct space analyses). Frequently, they are working with artificially created 2D periodic patterns, e.g. wallpapers, textiles, or building decoration in the Moorish/Arabic/Islamic tradition. The goals of these researchers are often related to the identification of point and translation symmetries by computational means and the subsequent classifications of patterns into groups. Since their patterns were artificially created, they do not need to obey all of the restrictions that nature typically imposes on long range periodic ordered arrays of atoms or molecules.
Computational geometry takes a broader view on this issue and concluded already in 1991 that the problem of testing approximate point symmetries in noisy images is in general NP-hard[29] and later on that it is also NP-complete. For restricted versions of this problem, there exist polynomial time algorithms that solve the corresponding optimization problems for a few point symmetries in 2D.[30]
Crystallographic image processing of high-resolution TEM images
The principal steps for solving a structure of an inorganic crystal from HREM images by CIP are as follows (for a detailed discussion see [31]).
- Selecting the area of interest and calculation of the Fourier transform (= power spectrum consisting of a 2D periodic array of complex numbers)
- Determining the defocus value and compensating for the contrast changes imposed by the objective lens (done in Fourier space)
- Indexing and refining the lattice (done in Fourier space)
- Extracting amplitudes and phase values at the refined lattice positions (done in Fourier space)
- Determining the origin of the projected unit cell and determining the projected (plane group) symmetry
- Imposing constrains of the most likely plane group symmetry on the amplitudes an phases. At this step the image phases are converted into the phases of the structure factors.
- Calculating the pseudo-potential map by Fourier synthesis with corrected (structure factor) amplitudes and phases (done in real space)
- Determining 2D (projected) atomic co-ordinates (done in real space)
A few computer programs are available which assist to perform the necessary steps of processing. The most popular programs used by materials scientists (electron crystallographers) are CRISP,[32][33][34] VEC,[35][36] and the EDM package.[37] There is also the recently developed crystallographic image processing program EMIA,[38] but so far there do not seem to be reports by users of this program.
Structural biologists achieve resolutions of a few ångströms (up from a to few nanometers in the past when samples used to be negatively stained) for membrane forming proteins in regular two-dimensional arrays, but prefer the usage of the programs 2dx,[39] EMAN2,[40] and IPLT.[41] These programs are based on the Medical Research Council (MRC) image processing programs [42][43][44] and possess additional functionality such as the "unbending" [45][46] of the image. As the name suggests, unbending of the image is conceptually equivalent to "flattening out and relaxing to equilibrium positions" one building block thick samples so that all 2D periodic motifs are as similar as possible and all building blocks of the array possess the same crystallographic orientation with respect to a cartesian coordinate system that is fixed to the microscope. (The microscope's optical axis typically serves as the z-axis.) Unbending is often necessary when the 2D array of membrane proteins is paracrystalline rather than genuinely crystalline. It was estimated that unbending approximately doubles the spatial resolution with which the shape of molecules can be determined[47]
Inorganic crystals are much stiffer than 2D periodic protein membrane arrays so that there is no need for the unbending of images that were taken from suitably thinned parts of these crystals. Consequently, the CRISP program does not possess the unbending image processing feature but offers superior performance in the so-called phase origin refinement.
The latter feature is particularly important for electron crystallographers as their samples may possess any space group out of the 230 possible groups types that exist in three dimensions. The regular arrays of membrane forming proteins that structural biologists deal with are, on the other hand, restricted to possess one out of only 17 (two-sided/black-white) layer group types (of which there are 46 in total and which are periodic only in 2D) due to the chiral nature of all (naturally occurring) proteins. Different crystallographic settings of four of these layer group types increase the number of possible layer group symmetries of regular arrays of membrane forming proteins to just 21.
All 3D space groups and their subperiodic 2D periodic layer groups (including the above-mentioned 46 two-sided groups) project to just 17 plane space group types, which are genuinely 2D periodic and are sometimes referred to as the wallpaper groups. (Although quite popular, this is a misnomer because wallpapers are not restricted to possess these symmetries by nature.)
All individual transmission electron microscopy images are projections from the three-dimensional space of the samples into two dimensions (so that spatial distribution information along the projection direction is unavoidably lost). Projections along prominent (i.e. certain low-index) zone axes of 3D crystals or along the layer normal of a membrane forming protein sample ensure the projection of 3D symmetry into 2D. (Along arbitrary high-index zone axes and inclined to the layer normal of membrane forming proteins, there will be no useful projected symmetry in transmission images.) The recovery of 3D structures and their symmetries relies on electron tomography techniques, which use sets of transmission electron microscopy images.
The origin refinement part of CIP relies on the definition of the plane symmetry group types as provided by the International Tables of Crystallography, where all symmetry equivalent positions in the unit cell and their respective site symmetries are listed along with systematic absences in reciprocal space. Besides plane symmetry groups p1, p3, p3m1 and p31m, all other plane group symmetries are centrosymmetric so that the origin refinement simplifies to the determination of the correct signs of the amplitudes of the Fourier coefficients.
When crystallographic image processing is utilized in scanning probe microscopy, the symmetry groups to be considered are just the 17 plane space group types in their possible 21 settings.
Crystallographic processing of images that were recorded from 2D periodic arrays with other types of microscopes
Because digitized 2D periodic images are in the information theoretical approach just data organized in 2D arrays of pixels, core features of Crystallographic Image Processing can be utilized independent of the type of microscope with which the images/data were recorded. The CIP technique has, accordingly been applied (on the basis of the 2dx program) to atomic resolution Z-contrast images of Si-clathrates, as recorded in an aberration-corrected scanning transmission electron microscope.[48] Images of 2D periodic arrays of flat lying molecules on a substrate as recorded with scanning tunneling microscopes [49][50] were also crystallographic processed utilizing the program CRISP.
References
- T. E. Weirich, From Fourier series towards crystal structures - a survey of conventional methods for solving the phase problem; in: Electron Crystallography - Novel Approaches for Structure Determination of Nanosized Materials, T. E. Weirich, J. L. Lábár, X. Zou, (Eds.), Springer 2006, 235 - 257.
- Thust, A.; Overwijk, M.H.F.; Coene, W.M.J.; Lentzen, M. (1996). "Numerical correction of lens aberrations in phase-retrieval HRTEM". Ultramicroscopy. 64 (1–4): 249–264. doi:10.1016/0304-3991(96)00022-8.
- Allen, L.J.; McBride, W.; O'Leary, N.L.; Oxley, M.P. (2004). "Exit wave reconstruction at atomic resolution". Ultramicroscopy. 100 (1–2): 91–104. doi:10.1016/j.ultramic.2004.01.012. PMID 15219694.
- Barthel, Juri; Weirich, Thomas E.; Cox, Gerhard; Hibst, Hartmut; Thust, Andreas (2010). "Structure of Cs0.5[Nb2.5W2.5O14] analysed by focal-series reconstruction and crystallographic image processing". Acta Materialia. 58 (10): 3764–3772. Bibcode:2010AcMat..58.3764B. doi:10.1016/j.actamat.2010.03.016.
- Klug, A. (1979). "Image Analysis and Reconstruction in the Electron Microscopy of Biological Macromolecules". Chemica Scripta. 14: 245–256.
- Amos, L.A.; Henderson, Richard; Unwin, P.N.T. (1982). "Three-dimensional structure determination by electron microscopy of two-dimensional crystals". Progress in Biophysics and Molecular Biology. 39 (3): 183–231. doi:10.1016/0079-6107(83)90017-2. PMID 6289376.
- Hovmöller, Sven; Sjögren, Agneta; Farrants, George; Sundberg, Margareta; Marinder, Bengt-Olov (1984). "Accurate atomic positions from electron microscopy". Nature. 311 (5983): 238–241. Bibcode:1984Natur.311..238H. doi:10.1038/311238a0.
- Zou, Xiaodong; Sundberg, Margareta; Larine, Maxim; Hovmöller, Sven (1996). "Structure projection retrieval by image processing of HREM images taken under non-optimum defocus conditions" (PDF). Ultramicroscopy. 62 (1–2): 103–121. doi:10.1016/0304-3991(95)00090-9. PMID 22666921.
- Weirich, Thomas E.; Ramlau, Reiner; Simon, Arndt; Hovmöller, Sven; Zou, Xiaodong (1996). "A crystal structure determined with 0.02 Å accuracy by electron microscopy". Nature. 382 (6587): 144–146. Bibcode:1996Natur.382..144W. doi:10.1038/382144a0. S2CID 4327149.
- Downing, Kenneth H.; Meisheng, Hu; Wenk, Hans-Rudolf; O'Keefe, Michael A. (1990). "Resolution of oxygen atoms in staurolite by three-dimensional transmission electron microscopy". Nature. 348 (6301): 525–528. Bibcode:1990Natur.348..525D. doi:10.1038/348525a0. S2CID 4340756.
- Wenk, H.-R.; Downing, K. H.; Hu, M.; O'Keefe, M. A. (1992). "3D structure determination from electron-microscope images: Electron crystallography of staurolite". Acta Crystallographica Section A. 48 (5): 700–716. doi:10.1107/s0108767392000850. PMID 1445681.
- Zou, X. D.; Mo, Z. M.; Hovmöller, S.; Li, X. Z.; Kuo, K. H. (2003). "Three-dimensional reconstruction of the ν-AlCr Fe phase by electron crystallography" (PDF). Acta Crystallographica Section A. 59 (6): 526–539. doi:10.1107/S0108767303018051. PMID 14581752.
- Gramm, Fabian; Baerlocher, Christian; McCusker, Lynne B.; Warrender, Stewart J.; Wright, Paul A.; Han, Bada; Hong, Suk Bong; Liu, Zheng; Ohsuna, Tetsu; Terasaki, Osamu (2006). "Complex zeolite structure solved by combining powder diffraction and electron microscopy". Nature. 444 (7115): 79–81. Bibcode:2006Natur.444...79G. doi:10.1038/nature05200. PMID 17080087. S2CID 4396820.
- Sun, Junliang; He, Zhanbing; Hovmöller, Sven; Zou, Xiaodong; Gramm, Fabian; Baerlocher, Christian; McCusker, Lynne B. (2010). "Structure determination of the zeolite IM-5 using electron crystallography" (PDF). Zeitschrift für Kristallographie. 225 (2–3): 77–85. Bibcode:2010ZK....225...77S. doi:10.1524/zkri.2010.1204. S2CID 98380733.
- Y. Liu, "Computational Symmetry" in: Symmetry 2000, Part I, eds. I. Hargittai and T. C. Laurent, chapter 21, p. 231–245, Portland Press, London, 2002, (Wenner-Gren International Series, vol. 80), https://www.cs.cmu.edu/~yanxi/images/computationalSymmetry.pdf
- Y. Liu, H. Hel-Or, C. S. Kaplan, and L. V. Gool (2009) "Foundations and Trends in Computer Graphics and Vision" vol. 5, Nos. 1–2, p. 1–195, open access: http://vision.cse.psu.edu/publications/pdfs/liuCSinCV.pdf, also published as a book by Now Publishers Inc., Boston and Delft, 2010
- Zabrodsky, H.; Peleg, S.; Avnir, D. (1995). "Symmetry as a continuous feature" (PDF). IEEE Transactions on Pattern Analysis and Machine Intelligence. 17 (12): 1154–1166. doi:10.1109/34.476508. S2CID 13894016.
- Y. Liu (2014) "Computational Symmetry", Computer Vision, A Reference Guide, Ikeuchi, K. (ed.), Springer, 2014
- Albert, F.; Gómis, J.M.; Blasco, J.; Valiente, J.M.; Aleixos, N. (2015). "A new method to analyse mosaics based on Symmetry Group theory applied to Islamic Geometric Patterns". Computer Vision and Image Understanding. 130: 54–70. doi:10.1016/j.cviu.2014.09.002. hdl:10251/51095. S2CID 207060370.
- Hahn T. (2005) International Tables for Crystallography, Brief Teaching Edition of Volume A, Space-group symmetry. 5th revised edition, Chester: International Union of Crystallography
- Kanatani, K. (1997). "Comments on "Symmetry as a Continuous Feature"". IEEE Transactions on Pattern Analysis and Machine Intelligence. 19 (3): 246–247. doi:10.1109/34.584101.
- K. Kanatani, (1996) Statistical Optimization for Geometric Computation: Theory and Practice, Dover Books on Mathematics, Mineola, New York
- K. P. Burnham and D R Anderson (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edition. Springer, New York
- Burnham, Kenneth P.; Anderson, David R.; Huyvaert, Kathryn P. (2011). "AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons". Behavioral Ecology and Sociobiology. 65: 23–35. doi:10.1007/s00265-010-1029-6. S2CID 3354490.
- Kanatani, Kenichi (1998). "Geometric Information Criterion for Model Selection". International Journal of Computer Vision. 26 (3): 171–189. doi:10.1023/A:1007948927139. S2CID 19248991.
- Yanxi Liu; Collins, R.T.; Tsin, Y. (2004). "A computational model for periodic pattern perception based on frieze and wallpaper groups". IEEE Transactions on Pattern Analysis and Machine Intelligence. 26 (3): 354–371. doi:10.1109/TPAMI.2004.1262332. PMID 15376882. S2CID 2394019.
- Straton, Jack C.; Bilyeu, Taylor T.; Moon, Bill; Moeck, Peter (2014). "Double-tip effects on scanning tunneling microscopy imaging of 2D periodic objects: Unambiguous detection and limits of their removal by crystallographic averaging in the spatial frequency domain". Crystal Research and Technology. 49 (9): 663–680. doi:10.1002/crat.201300240. S2CID 94543563.
- Straton, Jack C.; Moon, Bill; Bilyeu, Taylor T.; Moeck, Peter (2015). "Removal of multiple-tip artifacts from scanning tunneling microscope images by crystallographic averaging". Advanced Structural and Chemical Imaging. 1. doi:10.1186/s40679-015-0014-6. S2CID 51783244.
- Iwanowski, Sebastian (1991). "Testing approximate symmetry in the plane is NP-hard". Theoretical Computer Science. 80 (2): 227–262. doi:10.1016/0304-3975(91)90389-J.
- C. Dieckmann (2012) Symmetry Detection and Approximation, Dissertation zur Erlangung des Doktorgrades, Fachbereich Mathematik und Informatik der Freien Universität Berlin
- X.D. Zou, T.E. Weirich & S. Hovmöller (2001) "Electron Crystallography - Structure determination by combining HREM, crystallographic image processing and electron diffraction." In: Progress in Transmission Electron Microscopy, I. Concepts and Techniques, X.F. Zhang, Z. Zhang Ed., Springer Series in Surface Science. Vol. 38, Springer 2001, 191 – 222. ISBN 978-3-540-67681-2
- Hovmöller, Sven (1992). "CRISP: Crystallographic image processing on a personal computer". Ultramicroscopy. 41 (1–3): 121–135. doi:10.1016/0304-3991(92)90102-P.
- Hovmöller, Sven; Sukharev, Yuri I.; Zharov, Andrei G. (1991). "CRISP — A new system for crystallographic image processing on personal computers". Micron and Microscopica Acta. 22 (1–2): 141–142. doi:10.1016/0739-6260(91)90130-R.
- Zhang, H.; Yu, T.; Oleynikov, P.; Zhao, D.Y.; Hovmöller, S.; Zou, X.D. (2007). "Crisp and e Map: Software for determining 3D pore structures of ordered mesoporous materials by electron crystallography". Recent Progress in Mesostructured Materials - Proceedings of the 5th International Mesostructured Materials Symposium (IMMS2006), Shanghai, P.R. China, August 5-7, 2006. Studies in Surface Science and Catalysis. Vol. 165. pp. 109–112. doi:10.1016/S0167-2991(07)80277-1. ISBN 9780444521781.
- Wan, Z.; Liu, Y.; Fu, Z.; Li, Y.; Cheng, T.; Li, F.; Fan, H. (2003). "Visual computing in electron crystallography". Zeitschrift für Kristallographie - Crystalline Materials. 218 (4): 308–315. Bibcode:2003ZK....218..308W. doi:10.1524/zkri.218.4.308.20739. S2CID 96860861.
- Xue-Ming, Li; Fang-Hua, Li; Hai-Fu, Fan (2009). "A revised version of the program VEC (Visual computing in electron crystallography)". Chinese Physics B. 18 (6): 2459–2463. Bibcode:2009ChPhB..18.2459L. doi:10.1088/1674-1056/18/6/056. S2CID 250873737.
- Kilaas, R.; Marks, L.D.; Own, C.S. (2005). "EDM 1.0: Electron direct methods". Ultramicroscopy. 102 (3): 233–237. doi:10.1016/j.ultramic.2004.10.004. PMID 15639355.
- "Home". analitex.com.
- Gipson, Bryant; Zeng, Xiangyan; Zhang, Zi Yan; Stahlberg, Henning (2007). "2dx—User-friendly image processing for 2D crystals". Journal of Structural Biology. 157 (1): 64–72. doi:10.1016/j.jsb.2006.07.020. PMID 17055742.
- "EMAN2 - EMAN Wiki".
- Philippsen, A.; Schenk, A. D.; Stahlberg, H.; Engel, A. (2003). "Iplt--image processing library and toolkit for the electron microscopy community". Journal of Structural Biology. 144 (1–2): 4–12. doi:10.1016/j.jsb.2003.09.032. PMID 14643205.
- Crowther, R.A.; Henderson, R.; Smith, J.M. (1996). "MRC Image Processing Programs". Journal of Structural Biology. 116 (1): 9–16. doi:10.1006/jsbi.1996.0003. PMID 8742717.
- "Image Processing Software".
- "Home". ccpem.ac.uk.
- Gil, Debora; Carazo, Jose Maria; Marabini, Roberto (2006). "On the nature of 2D crystal unbending". Journal of Structural Biology. 156 (3): 546–555. doi:10.1016/j.jsb.2006.07.012. PMID 16997575.
- Henderson, R.; Baldwin, J.M.; Downing, K.H.; Lepault, J.; Zemlin, F. (1986). "Structure of purple membrane from halobacterium halobium: Recording, measurement and evaluation of electron micrographs at 3.5 Å resolution". Ultramicroscopy. 19 (2): 147–178. doi:10.1016/0304-3991(86)90203-2.
- Braun, Thomas; Engel, Andreas (2005). "Two‐dimensional Electron Crystallography". eLS. doi:10.1038/npg.els.0003044. ISBN 9780470016176.
- Morgan, D. G.; Ramasse, Q. M.; Browning, N. D. (2009). "Application of two-dimensional crystallography and image processing to atomic resolution Z-contrast images". Journal of Electron Microscopy. 58 (3): 223–244. doi:10.1093/jmicro/dfp007. PMID 19297343.
- Moeck, P., Toader, M., Abdel-Hafiez, M., Michael Hietschold, M. (2009) "Quantifying and enforcing two-dimensional symmetries in scanning probe microscopy images", in: "Frontiers of Characterization and Metrology for Nanoelectronics", edited by D. G. Seiler, A. C. Diebold, R. McDonald, C. M. Garner, D. Herr, R. P. Khosla, and E. M. Secula, American Institute of Physics, 978-0-7354-0712-1/09.
- Moeck, P. (2011) "Crystallographic Image Processing for Scanning Probe Microscopy" In: “Microscopy: Science Technology, Applications and Education”, Microscopy Book Series No. 4, Vol. 3, pp. 1951-1962, A. Méndez-Vilas and J. Diaz (editors), Formatex Research Center, 2010, ISBN 978-84-614-6191-2, arXiv:2011.13102
External links
- MRC Image Processing programs, the classical standard for structural biology, free for academic usages (Fortran source code)
- CRISP commercial, but superior for inorganic electron crystallography (for Windows PCs)
- VEC free for academic usages, particularly useful for the analysis of incommensurately modulated structures (for Windows PCs)
- IPLT open source, structural biology (for Mac PCs, Linux, and a Windows PC demo version)
- EMAN Vers. 2, open source, structural biology including single particle reconstruction (Linux)
- 2dx open source, mainly for structural biology (for Mac PCs, Linux)
- EDM open source, free for non-commercial purposes - a ready to go version of EDM is implemented on the elmiX Linux live CD