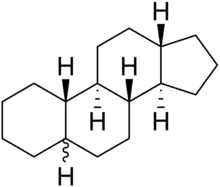
Gonane
Gonane (cyclopentanoperhydrophenanthrene) is a chemical compound with formula C
17H
28, whose structure consists of four hydrocarbon rings fused together: three cyclohexane units and one cyclopentane. It can also be viewed as the result of fusing a cyclopentane molecule with a fully hydrogenated molecule of phenanthrene, hence the more descriptive name perhydrocyclopenta[a]phenanthrene. The non-systematic version of the above name is cyclopentanoperhydrophenanthrene.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5ξ-Gonane | |
| Systematic IUPAC name
(3aR,3bS,5aΞ,9aS,9bR,11aS)-Hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H28 | |
| Molar mass | 232.411 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gonane is a tetracyclic hydrocarbon with no double bonds. It is formally the parent compound of the steroids; its carbon skeleton is called the "steroid nucleus".[1][2][3] Some important gonane derivatives are the steroid hormones, characterized by methyl groups at the C10 and C13 positions and a side chain at the C17 position.[3]
Because gonane has six centers of chirality, it has 64 (26) theoretically possible stereoisomers,[2] that differ on the position of the lone hydrogens at carbons 5, 8, 9, 10, 13 and 14 in the direction perpendicular to the mean plane of the carbons. However, only a few of these stereoisomers occur in living organisms.[2] The most common are 5α-gonane and 5β-gonane.
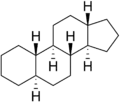 5α-Gonane
5α-Gonane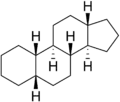 5β-Gonane
5β-Gonane
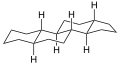 5α-Gonane, side-perspective view
5α-Gonane, side-perspective view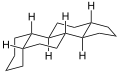 5β-Gonane, side-perspective view
5β-Gonane, side-perspective view
Estrane (C18) is the 13β-methyl variant of gonane, androstane (C19) is the 10β,13β-dimethyl variant of gonane, and pregnane (C21) is the 10β,13β-dimethyl, 17β-ethyl variant of gonane.[4][5]
The term gonane is also used to refer to a group of progestins that are carbon 18-homologated 19-nortestosterone derivatives including levonorgestrel and its analogues.[6] The term is used to distinguish them from the estranes (19-nortestosterone derivatives).[6]
References
- Yang, Yanqing; Krin, Anna; Cai, Xiaoli; Poopari, Mohammad Reza; Zhang, Yuefei; Cheeseman, James R.; Xu, Yunjie (2023-01-12). "Conformations of Steroid Hormones: Infrared and Vibrational Circular Dichroism Spectroscopy". Molecules (Basel, Switzerland). 28 (2): 771. doi:10.3390/molecules28020771. ISSN 1420-3049. PMC 9864676. PMID 36677830.
- Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9.
- James G. Speight (24 December 2010). Handbook of Industrial Hydrocarbon Processes. Gulf Professional Publishing. pp. 474–. ISBN 978-0-08-094271-1.
- D. Sriram (1 September 2010). Medicinal Chemistry. Pearson Education India. pp. 594–. ISBN 978-81-317-3144-4.
- Etienne-Emile Baulieu; Paul A. Kelly (30 November 1990). Hormones: From Molecules to Disease. Springer Science & Business Media. pp. 391–. ISBN 978-0-412-02791-8.
- Edgren RA, Stanczyk FZ (December 1999). "Nomenclature of the gonane progestins". Contraception. 60 (6): 313. doi:10.1016/s0010-7824(99)00101-8. PMID 10715364.