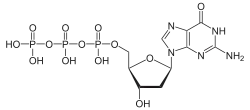
Deoxyguanosine triphosphate
Deoxyguanosine triphosphate[1] (dGTP) is a nucleoside triphosphate, and a nucleotide precursor used in cells for DNA synthesis. The substance is used in the polymerase chain reaction technique, in sequencing, and in cloning. It is also the competitor of inhibition onset by acyclovir in the treatment of HSV virus.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2′-Deoxyguanosine 5′-(tetrahydrogen triphosphate) | |
| Systematic IUPAC name
O1-{[(2R,3S,5R)-5-(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3-hydroxyoxolan-2-yl]methyl} tetrahydrogen triphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.080 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16N5O13P3 | |
| Molar mass | 507.181023 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Nelson, David L.; Cox, Michael M. (2005). Principles of Biochemistry (4th ed.). New York: W. H. Freeman. ISBN 0-7167-4339-6.
- Furman PA, Lambe CU, Nelson DJ (July 1982). "Effect of acyclovir on the deoxyribonucleoside triphosphate pool levels in Vero cells infected with herpes simplex virus type 1". Am. J. Med. 73 (1A): 14–7. doi:10.1016/0002-9343(82)90056-0. PMID 6285704.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.