Coenzyme F420
Coenzyme F420 or 8-hydroxy-5-deazaflavin is a coenzyme (sometimes called a cofactor) involved in redox reactions in methanogens,[1] in many Actinomycetota, and sporadically in other bacterial lineages. It is a flavin derivative with an absorption maximum at 420 nm—hence its name. The coenzyme is a substrate for coenzyme F420 hydrogenase,[2] 5,10-methylenetetrahydromethanopterin reductase and methylenetetrahydromethanopterin dehydrogenase.[3][4]
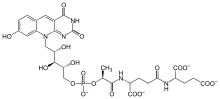
A particularly rich natural source of F420 is Mycobacterium smegmatis, in which several dozen enzymes use F420 instead of the related cofactor FMN used by homologous enzymes in most other species.[5] Eukaryotes including the fruit fly Drosophila melanogaster and the algae Ostreococcus tauri also use a precursor to this cofactor.[6]
Biosynthesis
Coenzyme F420 is synthesized via a multi-step pathway:
- 7,8-didemethyl-8-hydroxy-5-deazariboflavin synthase produces Coenzyme FO (also written F0), itself a cofactor of DNA photolyase (antenna). This is the head portion of the molecule.[6]
- 2-phospho-L-lactate transferase produces Coenzyme F420-0, the portion containing the head, the diphosphate bridge, and ending with a carboxylic acid group.
- Coenzyme F420-0:L-glutamate ligase puts a glutamate residue at the -COOH end, producing Coenzyme F420-1.
- Coenzyme F420-1:gamma-L-glutamate ligase puts a gamma-glutamate residue at the -COOH end, producing Coenzyme F420-2, the final compound (in its oxidized form).
References
- Deppenmeier U (September 2002). "Redox-driven proton translocation in methanogenic Archaea". Cellular and Molecular Life Sciences. 59 (9): 1513–33. doi:10.1007/s00018-002-8526-3. PMID 12440773. S2CID 23199201.
- Fox JA, Livingston DJ, Orme-Johnson WH, Walsh CT (July 1987). "8-Hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum: 1. Purification and characterization". Biochemistry. 26 (14): 4219–27. doi:10.1021/bi00388a007. PMID 3663585.
- Hagemeier CH, Shima S, Thauer RK, Bourenkov G, Bartunik HD, Ermler U (October 2003). "Coenzyme F420-dependent methylenetetrahydromethanopterin dehydrogenase (Mtd) from Methanopyrus kandleri: a methanogenic enzyme with an unusual quarternary structure". Journal of Molecular Biology. 332 (5): 1047–57. doi:10.1016/S0022-2836(03)00949-5. PMID 14499608.
- te Brömmelstroet BW, Geerts WJ, Keltjens JT, van der Drift C, Vogels GD (September 1991). "Purification and properties of 5,10-methylenetetrahydromethanopterin dehydrogenase and 5,10-methylenetetrahydromethanopterin reductase, two coenzyme F420-dependent enzymes, from Methanosarcina barkeri". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1079 (3): 293–302. doi:10.1016/0167-4838(91)90072-8. PMID 1911853.
- Selengut JD, Haft DH (November 2010). "Unexpected abundance of coenzyme F(420)-dependent enzymes in Mycobacterium tuberculosis and other actinobacteria". Journal of Bacteriology. 192 (21): 5788–98. doi:10.1128/JB.00425-10. PMC 2953692. PMID 20675471.
- Glas AF, Maul MJ, Cryle M, Barends TR, Schneider S, Kaya E, Schlichting I, Carell T (July 2009). "The archaeal cofactor F0 is a light-harvesting antenna chromophore in eukaryotes". Proceedings of the National Academy of Sciences of the United States of America. 106 (28): 11540–5. doi:10.1073/pnas.0812665106. PMC 2704855. PMID 19570997.
External links
- KEGG: