Dehydrogenative coupling of silanes
The dehydrogenative coupling of silanes is a reaction type for the formation of Si-Si bonds. Although never commercialized, the reaction has been demonstrated for the synthesis of certain disilanes as well as polysilanes. These reactions generally require catalysts.
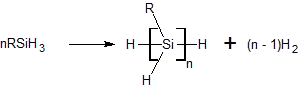
Metallocene-based catalysts
Titanocene and related their complexes are typical catalysts. A typical reaction involves phenylsilane:[1][2]
- n PhSiH3 → [PhSiH]n + n H2
Para- and meta-substituted phenylsilanes polymerize readily but ortho-substituted polymers were failed to form. Polymers white/colorless, tacky and soluble in organic solvents. Crosslinking was not observed.[3]
Using Cp2Ti(OPh)2 as a catalyst, the dehydrogenative coupling of phenylsilane in the presence of vinyltriethoxysilane produces a polysilane terminated with a triethoxysilylvinyl group.[4]
Other catalysts
The nickel(I) complex [(dippe)Ni(µ-H)]2 promotes the dehydrogenative coupling of some silanes.[5] While catalysts for dehydrogenative coupling reactions generally tend to be transition metal complexes, magnesium oxide and calcium oxide promote the dehydrogenation of phenylsilane. Being a heterogeneous process, the products are easily separated from the catalyst.[6] Dehydrogenative coupling of primary silanes using Wilkinson's catalyst is slow and dependent on the removal of H2 product. This conversion proceeds by oxidative addition of the Si-H bond and elimination of dihydrogen.[7] Tris(pentafluorophenyl)borane (B(C6F5)3)) is yet another catalyst for the dehydrogenative coupling of tertiary silanes. This system has the useful characteristic of being selective for Si-H bonds vs Si-Si bonds, leading to fewer branches and more linear polymers. This catalyst is particularly useful in reactions involving thiols and tertiary silanes or disilanes.[8]
Related reactions of hydrosilanes
As well as being coupled to each other, tertiary silanes can be coupled with carboxylic acids to form silyl esters. Ru3(CO)12/EtI is a good catalyst for this. This reaction applies to a wide range of silanes and acids.[9] The complex [Cu(PPh3)3Cl] can also be used to produce silyl esters.[10]
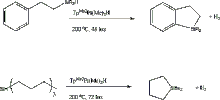
Tertiary silanes may also be dehydrogenatively coupled to aromatic rings with the use of the catalyst TpMe2Pt(Me)2H (TpMe2 = hydrido tris(3,5-dimethylpyrazolyl)borate). For example, this platinum catalyst can be used to react triethyl silane with benzene to produce phenyltriethylsilane, with the elimination of hydrogen gas. This is a terrific catalyst because it eliminates the need for a hydrogen acceptor, something which is normally required for the silation of a C-H bond. This reaction may also be done intramolecularly to produce five- or six-membered silicon-containing rings fused to a phenyl ring. In addition, tributylsilane can be converted into the corresponding cyclic organosilane via the same process. A drawback to this catalyst, however, is that it requires rather harsh reaction conditions (typically 200 °C for 24 hours for the intermolecular reaction, 48 – 72 hours for the intramolecular ones). It is also not particularly regioselective, so starting materials containing substituted benzene would result in a mixture of products.[11]
Polymer characterization
1H and 29Si NMR spectroscopy can sometimes be used to help identify and characterize products from these reactions.[1]
Infrared spectroscopy may also be useful as it can indicate whether or not the product is a tertiary silane. The stretch for Si-H is seen at around 2100 and 910 cm−1. In the case of tertiary silanes however, the peak at 910 cm−1 is not seen. The shift or change in these peaks will be affected by the size of the polymer.[1]
References
- Aitken, C.; Harrod, J. F.; Gill, U. S. (1987). "Structural studies of oligosilanes produced by catalytic dehydrogenative coupling of primary organosilanes". Can. J. Chem. 65: 1804–1809. doi:10.1139/v87-303.
- Corey, J.Y.; Zhu, X.H.; Bedard, T.C.; Lange, L.D. (1991). "Catalytic Dehydrogenative Coupling of Secondary Silanes with Cp2MCl2". Organometallics. 10 (4): 924. doi:10.1021/om00050a024.
- Banovetz, John P.; Suzuki, Hiroshi; Waymouth, Robert M. (1993). "Dehydrogenative coupling of substituted phenylsilanes synthesis of poly[((trifluoromethyl)phenyl)silanes]". Organometallics. 12 (11): 4700–4703. doi:10.1021/om00035a070.
- Garcia, Julien; Meyer, Daniel J.M.; Guillaneux, Denis; Moreau, Joël J.E.; Wong Chi Man, Michel (July 2009). "Investigation of titanium-catalysed dehydrogenative coupling and hydrosilylation of phenylhydrogenosilanes in a one-pot process". Journal of Organometallic Chemistry. 694 (15): 2427–2433. doi:10.1016/j.jorganchem.2009.03.018.
- Smith, Erin E.; Du, Guodong; Fanwick, Phillipe E.; Abu-Omar, Mahdi M. (2010). "Dehydrocoupling of Organosilanes with a Dinuclear Nickel Hydride Catalyst and Isolation of a Nickel Silyl Complex". Organometallics. 29 (23): 6529. doi:10.1021/om100887v.
- Itoh, M.; Mitsuzuka, M.; Utsumi, T.; Iwata, K.; Inoue, K. (1994). "Dehydrogenative coupling reactions between hydrosilanes and monosubstituted alkynes catalyzed by solid bases". Journal of Organometallic Chemistry. 476: C30–C31. doi:10.1016/0022-328X(94)87091-8.
- Rosenberg, Lisa; Kobus, Danielle N. (2003). "Dehydrogenative coupling of primary alkyl silanes using Wilkinson's catalyst". Journal of Organometallic Chemistry. 685 (1–2): 107–112. doi:10.1016/S0022-328X(03)00712-5.
- Harrison, D. J.; Edwards, D. R.; McDonald, R.; Rosenberg, L. (2008). "Toward selective functionalisation of oligosilanes: borane-catalysed dehydrogenative coupling of silanes with thiols". Dalton Trans. 26: 3401–3411. doi:10.1039/b806270f.
- Liu, G.; Zhao, H. (2008). "Ru-catalyzed dehydrogenative coupling of carboxylic acids and silanes - a new method for the preparation of silyl esters". Beilstein Journal of Organic Chemistry. 4 (27). doi:10.3762/bjoc.4.27. PMC 2511026.
- Liu, G. -B.; Zhao, H. -Y.; Thiemann, T. (2007). "Two New Catalysts for the Dehydrogenative Coupling Reaction of Carboxylic Acids with Silanes - Convenient Methods for an Atom-Economical Preparation of Silyl Esters". Synthetic Communications. 37: 2717–2727. doi:10.1080/00397910701465669.
- Tsukada, N.; Hartwig, J. F. (2005). "Intermolecular and intramolecular, platinum-catalyzed, acceptorless dehydrogenative coupling of hydrosilanes with aryl and aliphatic methyl C-H bonds". Journal of the American Chemical Society (127): 5022–5023. doi:10.1021/ja050612p.
- Sakomto, Kenkichi; Yoshida, Masaru; Sakurai, Hideki (1990). "Highly ordered high-molecular weight alternating polysilylene copolymer prepared by anionic polymerization of masked disilene". Macromolecules. 23 (20): 4494–4496. doi:10.1021/ma00222a031.
- Shono, T.; Ishifune, S.; Nishida, R. (1997). "Electroreductive synthesis of some functionalized polysilanes and related polymers". Tetrahedron Letters. 38 (36): 4607–4610. doi:10.1016/S0040-4039(97)00987-8.