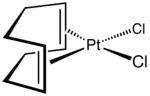
Dichloro(cycloocta-1,5-diene)platinum(II)
Dichloro(1,5-cyclooctadiene)platinum(II) (Pt(cod)Cl2) is an organometallic compound of platinum. This colourless solid is an entry point to other platinum compounds through the displacement of the cod and/or chloride ligands. It is one of several complexes of cycloocta-1,5-diene.
 | |
platinum(II)-from-xtal-3D-balls-A.png.webp) | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.937 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H12Cl2Pt | |
| Molar mass | 374.17 g·mol−1 |
| Melting point | 285 °C (545 °F; 558 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dichloro(1,5-cyclooctadiene)platinum(II) is prepared by treating potassium tetrachloroplatinate with the diene:[1]
- K2PtCl4 + C8H12 → PtCl2C8H12 + 2 KCl

Sample of Pt(cod)Cl2.
According to X-ray crystallography, the complex is square planar.[2][3]
References
- E. Costa; P. G. Pringle; M. Ravetz (1997). "[(1,2,5,6-η)-1,5-Cyclooctadiene]Dimethyl-Platinum(II)". Inorg. Synth. 31: 284–286. doi:10.1002/9780470132623.ch49. ISBN 978-0-470-13262-3.
- Anil B. Goel; Sarla Goel; Don Van Der Veer (1982). "Chemistry of Metal diene complexes: X-ray crystal structure of dichloro(1,5-cyclooctadine)platinum(II)". Inorganica Chimica Acta. 65: L205–L206. doi:10.1016/S0020-1693(00)93548-5.
- Ashfaquzzaman Syed; Edwin D. Stevens; Sandra G. Cruz (1984). "Reexamination of the π bonding in dichloro(cycloocta-1,5-diene)platinum". Inorg. Chem. 23 (22): 3673–3674. doi:10.1021/ic00190a053.
Further reading
- J. L. Butikofer, E. W. Kalberer, W. C. Schuster, and D. M. Roddick, "The Crystal Structure of Dichloro(norbornadiene)platinum(II): A Comparison to Dichloro(cyclooctadiene)platinum(II)," Acta Crystallogr. C. 2004, m353-m354.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.