Diethylmercury
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous.[1] This chemical can cross the blood–brain barrier, causing permanent brain damage. It is, however, considerably less toxic than dimethylmercury.
 | |
| Names | |
|---|---|
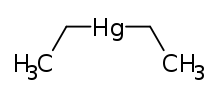
| IUPAC name
diethylmercury | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.001 |
| EC Number |
|
| MeSH | C007378 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C 4H 10Hg (C 2H 5) 2Hg | |
| Molar mass | 258.71 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet |
| Density | 2.446 g/ml |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 156 to 157 °C (313 to 315 °F; 429 to 430 K) |
| Insoluble | |
| Solubility | Ethers, hydrocarbons, THF |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Flammable, extremely toxic |
| GHS labelling: | |
   | |
| Danger | |
| H225, H300+H310+H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| Flash point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Diethylmercury can be obtained from the reaction between ethylmagnesium bromide and mercury(II) chloride.[2]
- 2 C2H5MgBr + HgCl2 → Hg(C2H5)2 + MgBr2 + MgCl2
Other methods are also known.[3]
See also
- Dimethylmercury, a related compound
- Ethylmercury
- Mercury poisoning
References
- "Diethyl Mercury | 627-44-1".
- Brauer, Georg. Handbuch der präparativen anorganischen Chemie Bd. 2. Baudler, Marianne (3rd ed.). Stuttgart. p. 1063. ISBN 978-3-432-87813-3. OCLC 310719490.
- Kolbe, Hermann (1860). Ausführliches Lehrbuch der organischen Chemie, Volume 2. p. 964.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.