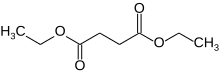
Diethyl succinate
Diethyl succinate is the diethyl ester of succinate.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Diethyl butanedioate | |
| Other names
Diethyl succinate Butanedioic acid diethyl ester Clorius | |
| Identifiers | |
3D model (JSmol) |
|
| 907645 | |
| ChemSpider | |
| ECHA InfoCard | 100.004.194 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H14O4 | |
| Molar mass | 174.196 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.047 g/mL |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Slightly soluble | |
| Vapor pressure | 0.13 mmHg |
| -105.07·10−6 cm3/mol | |
| Thermochemistry | |
Std enthalpy of combustion (ΔcH⦵298) |
24.22 kJ/g |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Primary irritant |
| NFPA 704 (fire diamond) | |
| Flash point | 90.56 °C (195.01 °F; 363.71 K) |
| Explosive limits | 1.1-6.5% |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is a colorless liquid with the formula (CH2CO2Et)2 (Et = ethyl). The organic molecule contains two ester groups. This ester is a versatile chemical intermediate. A colorless liquid, diethyl succinate is formed by Fisher esterification of succinic acid and ethanol.
Reactions
Being a diester, diethyl succinate is a particularly versatile building block. It participates in acyloin condensation to give 2-hydroxycyclobutanone.[1] Via condensation with oxalate esters, it serves as a precursor to ketoglutaric acid.[2] It is a reagent in the Stobbe condensation.
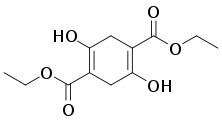
Diethylsuccinoylsuccinate, a useful precursor to dyes and pigments, is produced by base-induced condensation of diethyl succinate.[3]
References
- Bloomfield, Jordan J.; Nelke, Janice M. (1977). "Acyloin Condensation in Which Chlorotrimethylsilane is Used as a Trapping Agent: 1,2-Bis(Trimethylsilyloxy)Cyclobutene and 2-Hydroxycyclobutanone". Organic Syntheses. 57: 1. doi:10.15227/orgsyn.057.0001.
- Bottorff, E. M.; Moore, L. L. (1964). "α-Ketoglutaric Acid". Organic Syntheses. 44: 67. doi:10.15227/orgsyn.044.0067.
- Hunger, K.; Herbst, W. (2012). "Pigments, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_371.(subscription required)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
