Stobbe condensation
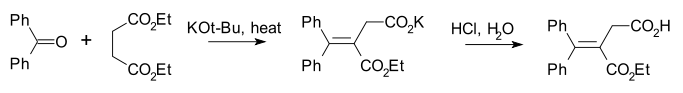
The Stobbe condensation entails the reaction of an aldehyde or ketone with an ester of succinic acid to generate alkylidene succinic acid or related derivatives.[1] The reaction consumes one equivalent of metal alkoxide. Commonly, diethylsuccinate is a component of the reaction. The usual product is salt of the half-ester. The Stobbe condensation is named after its discoverer, Hans Stobbe, whose work involved the sodium ethoxide-induced condensation of acetone and diethyl succinate.[2]
An example is the reaction of benzophenone with diethyl succinate:[3]
A reaction mechanism that explains the formation of both an ester group and a carboxylic acid group is centered on a lactone intermediate (5):
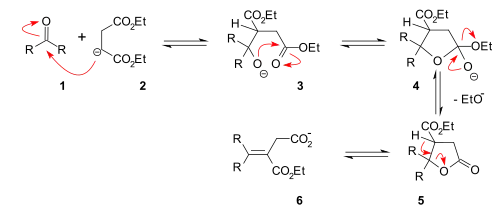 Reaction mechanism
Reaction mechanism
The Stobbe condensation is also illustrated by the synthesis of the drug tametraline.[4]
References
- Smith, Michael B.; March, Jerry (2006). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. p. 1452-1455. doi:10.1002/0470084960. ISBN 9780470084960.
- Stobbe, H. (1899). "Condensation des Benzophenons mit Bernsteinsäureester". Justus Liebigs Annalen der Chemie. 308 (1–2): 89–114. doi:10.1002/jlac.18993080106.
- Johnson, W. S.; Schneider, W. P. (1950). "β-Carbethoxy-γ,γ-Diphenylvinylacetic Acid". Organic Syntheses. 30: 18. doi:10.15227/orgsyn.030.0018.
- Sarges R (1975). "Synthesis of Phenyl-Substituted 1-Aminotetralines". The Journal of Organic Chemistry. 40 (9): 1216–1224. doi:10.1021/jo00897a008.
External links
- "Claisen Condensation". Organic Chemistry Portal.