Dinitrogen difluoride
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the azide FN3. It has the structure F−N=N−F and exists in both a cis- and trans-form.
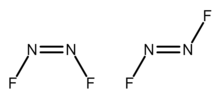 cis-Dinitrogen difluoride (left) and trans-dinitrogen difluoride (right) | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
cis- or trans-dinitrogen difluoride | |||
| Other names
cis- or trans-difluorodiazene | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| FN=NF | |||
| Molar mass | 66.011 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 2.698 g/L | ||
| Melting point | cis: less than −195 °C (−319.0 °F; 78.1 K) trans: −172 °C (−278 °F) | ||
| Boiling point | cis: −105.75 °C (−158.35 °F; 167.40 K) trans: −111.45 °C (−168.61 °F) | ||
| cis: 0.16 D trans: 0 D | |||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
cis: 69.5 kJ/mol trans: 82.0 kJ/mol | ||
| Related compounds | |||
Other anions |
Azide | ||
Other cations |
|||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Isomers
The cis configuration lies in a C2v symmetry and the trans-form has a symmetry of C2h. These isomers are thermally interconvertible but can be separated by low temperature fractionation. The trans-form is less thermodynamically stable but can be stored in glass vessels. The cis-form attacks glass over a time scale of about 2 weeks to form silicon tetrafluoride and nitrous oxide:[2]
- 2 N2F2 + SiO2 → SiF4 + 2 N2O
Preparation
Most preparations of dinitrogen difluoride give mixtures of the two isomers, but they can be prepared independently.
An aqueous method involves N,N-difluorourea with concentrated potassium hydroxide. This gives a 40% yield with three times more of the trans isomer.[3]
Difluoramine forms a solid unstable compound with potassium fluoride (or rubidium fluoride or caesium fluoride) which decomposes to dinitrogen difluoride.[3]
It can also be prepared by photolysis of tetrafluorohydrazine and bromine:[4]
- N2F4 N2F2 + byproducts
Reactions
The cis form of difluorodiazene will react with strong fluoride ion acceptors such as antimony pentafluoride to form the linear[5] [N≡N−F]+ cation (fluorodiazonium cation[5]) which forms a salt with the formula [N≡N−F]+[SbF6]− (fluorodiazonium hexafluoroantimonate(V)).
- F−N=N−F + SbF5 → [N≡N−F]+[SbF6]−
Analogous reaction of cis-difluorodiazene with arsenic pentafluoride gives white solid salt with the formula [N≡N−F]+[AsF6]−[5] (fluorodiazonium hexafluoroarsenate(V)).
- F−N=N−F + AsF5 → [N≡N−F]+[AsF6]−
In the solid phase, the observed N≡N and N−F bond distances in the [N≡N−F]+ cation are 1.089(9) and 1.257(8) Å respectively, among the shortest experimentally observed N-N and N-F bonds.
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 4–73, 5–15, 9–46. ISBN 0-8493-0594-2.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Sykes, A. G. (1989-07-17). Advances in Inorganic Chemistry. Academic Press. p. 171. ISBN 9780080578828. Retrieved 21 June 2014.
- Leon M. Zaborowski; et al. (1973), Aaron Wold and John K. Ruff (ed.), Chlorodifluoroamine and Difluorodiazene - B. Difluorodiazene (Dinitrogen difluoride), Inorganic Syntheses (in German), vol. 14, McGraw-Hill Book Company, Inc., pp. 34–39
- Cacace, Fulvio; Grandinetti, Felice; Pepi, Federico (1995). "Gaseous Fluorodiazonium Ions. Experimental and Theoretical Study on Formation and Structure of FN2+". Inorganic Chemistry. 34 (6): 1325–1332. doi:10.1021/ic00110a007.
-Dinitrogen-difluoride-3D-balls.png.webp)
-Dinitrogen-difluoride-3D-balls.png.webp)