Peroxynitrite
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate, NO−
3
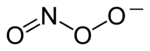 Chemical structure of the peroxynitrite anion | |
| Names | |
|---|---|
| IUPAC name
Oxido nitrite | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NO3− | |
| Molar mass | 62.005 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |

Preparation
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide:[1][2][3]
- NO + O−2 → NO(O2)−
It is prepared by the reaction of hydrogen peroxide with nitrite:[4]
- H2O2 + NO−
2 → ONOO− + H2O
Its presence is indicated by the absorbance at 302 nm (pH 12, ε302 = 1670 M−1 cm−1).
Reactions
Peroxynitrite is weakly basic with a pKa of ~6.8.
It is reactive toward DNA and proteins.
ONOO− reacts nucleophilically with carbon dioxide. In vivo, the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physiological conditions, the reaction of ONOO− with carbon dioxide to form nitrosoperoxycarbonate (ONOOCO−
2) is by far the predominant pathway for ONOO−. ONOOCO−
2 homolyzes to form carbonate radical and nitrogen dioxide, again as a pair of caged radicals. Approximately 66% of the time, these two radicals recombine to form carbon dioxide and nitrate. The other 33% of the time, these two radicals escape the solvent cage and become free radicals. It is these radicals (carbonate radical and nitrogen dioxide) that are believed to cause peroxynitrite-related cellular damage.
Peroxynitrous acid
Its conjugate acid peroxynitrous acid is highly reactive, although peroxynitrite is stable in basic solutions.[5][6]
See also
References
- Bohle, D. Scott; Sagan, Elisabeth S. (2004). "Tetramethylammonium Salts of Superoxide and Peroxynitrite". Inorganic Syntheses: 36. doi:10.1002/0471653683.ch1.
- Pacher, P; Beckman, J. S; Liaudet, L (2007). "Nitric oxide and peroxynitrite in health and disease". Physiological Reviews. 87 (1): 315–424. doi:10.1152/physrev.00029.2006. PMC 2248324. PMID 17237348.
- Szabó, C; Ischiropoulos, H; Radi, R (2007). "Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics". Nature Reviews Drug Discovery. 6 (8): 662–80. doi:10.1038/nrd2222. PMID 17667957.
- Beckman, J. S; Koppenol, W. H (1996). "Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly". American Journal of Physiology. Cell Physiology. 271 (5 Pt 1): C1424–37. doi:10.1152/ajpcell.1996.271.5.C1424. PMID 8944624.
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Koppenol, W. H (1998). "The chemistry of peroxynitrite, a biological toxin". Química Nova. 21 (3): 326–331. doi:10.1590/S0100-40421998000300014.