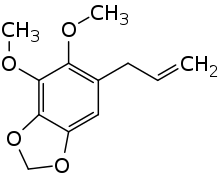
Dillapiole
Dillapiole is an organic chemical compound and essential oil commonly extracted from dill weed, though it can be found in a variety of other plants such as fennel root.[1] This compound is closely related to apiole, having a methoxy group positioned differently on the benzene ring.[2][3] Dillapiole works synergically with certain insecticides like pyrethrins similarly to piperonyl butoxide, which likely results from inhibition of the MFO enzyme of insects.[4]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4,5-Dimethoxy-6-(prop-2-en-1-yl)-2H-1,3-benzodioxole | |
| Other names
6-Allyl-4,5-dimethoxybenzo[d][1,3]dioxole 1-Allyl-2,3-dimethoxy-4,5-(methylenedioxy)benzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.149.911 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H14O4 | |
| Molar mass | 222.240 g·mol−1 |
| Density | 1.163 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
No carcinogenicity was detected with parsley apiol or dill apiol in mice.[5]
References
- Azeez, Shamina (2008). Chemistry of Spices. Calicut, Kerala, India: Biddles Ltd. pp. 227–241 [230]. ISBN 9781845934057.
- Santos, P. A. G.; Figueiredo, A. C.; Lourenço, P. M. L.; Barroso, J. G.; Pedro, L. G.; Oliveira, M. M.; Schripsema, J.; Deans, S. G.; Scheffer, J. J. C. (2002). "Hairy root cultures of Anethum graveolens (Dill): Establishment, growth, time-course study of their essential oil and its comparison with parent plant oils". Biotechnology Letters. 24 (12): 1031–1036. doi:10.1023/A:1015653701265. S2CID 10120732.
- Shulgin, A. T.; Sargent, T. (1967). "Psychotrophic phenylisopropylamines derived from apiole and dillapiole". Nature. 215 (5109): 1494–1495. Bibcode:1967Natur.215.1494S. doi:10.1038/2151494b0. PMID 4861200. S2CID 26334093.
- IN patent 128,129, Mankombu Sambasivan Swaminathan, "Improvements in or relating to methylenedioxyphenyl derivatives", published 1970-08-21, issued 1970-10-24
- Phillips, David H.; Reddy, M. Vijayaraj; Randerath, Kurt (1984). "32P-Post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. II. Newborn male B6C3F1 mice". Carcinogenesis. 5 (12): 1623–1628. doi:10.1093/carcin/5.12.1623. PMID 6499113.
See also
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.