Ductal cells
Ductal cells refer to the epithelial cell lining of the pancreatic duct that deliver enzymes from the acinar cells to the duodenum. They have the essential function of producing bicarbonate-rich (HCO3-) secretion to neutralize stomach acidity. The hormone secretin stimulates ductal cells and is responsible for maintaining the duodenal pH and preventing duodenal injury from acidic chyme. Ductal cells mix their production with acinar cells to make up the pancreatic juice.[1]
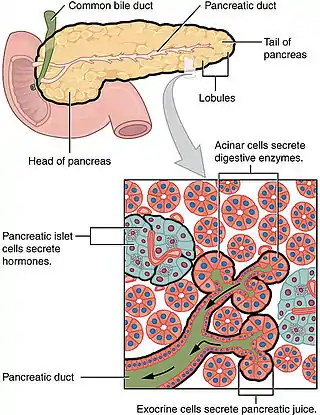
Ductal cells comprise about 10% of the pancreas by number and about 4% in volume. Its function is to secrete bicarbonate and mucins and to form the tubule network that transfers enzymes made by acinar cells to the duodenum. Ductal cells have a proliferation rate of about 0.5% in normal adults, but mitotic activity goes up when the pancreas is damaged.[3]
Ductal network
The ductal pancreas network originates from the central pancreatic duct—this main duct with the bile duct opens into the duodenum. The ductal cells of the main pancreatic duct are bound by connective tissue and produce a columnar epithelium.[3] Interlobular ducts originate from the main pancreatic duct and connect the various pancreatic lobes. In these lobes, the intercalated ducts expel acini. Meanwhile, the ductal cells of these intercalated ducts create a simple squamous epithelium that rapidly converts into simple cuboidal, and connective tissue also surrounds them.[3] As the ducts grow larger, the epithelium becomes cuboidal or columnar (when large in diameter, the ducts become stratified cuboidal), and connective tissue surrounds them. Pancreatic ductal cells are very similar to ductal cells of other exocrine glands (liver, bile duct, salivary glands).[3] Because of this, a common diagnosis affects these cells: cystic fibrosis.
Ductal cell physiology
While ductal cells are a minor type of cell in the adult pancreas, they have a critical function besides making the network that transfers enzymes from acini to the digestive tract. The primary function of pancreas ductal cells is to secrete a bicarbonate-rich, isotonic fluid. This fluid washes away the inactive form of digestive enzymes in the ductal system, neutralizes stomach acidity and mucins, and creates a pH environment necessary for the pancreas's normal function.[4]
Multiple factors affect the rate of bicarbonate secretion: species, cell location in the ductal system, secretory rate, etc. When stimulated, bicarbonate levels can get to 140mM. Due to this, there is a contrast in concentration between the outside and inside environment of ductal cells. The channels and ion transporters on ductal cells vary on the luminal and basolateral membrane, meaning there is functional polarization of the ductal cell.[4]
The largest network branches in this system contain goblet cells that interact with ductal cells, making up about 2% of this structure—these cells aid mucin assembly. Furthermore, unlike other exocrine glands, the pancreas does not have myoepithelial cells around the ducts.[3] Ductal cells have a single cilium that is made up of nine peripheral doublets but does not have a central microtubule. This cilium is considered vital for perceiving flow in ducts.[3]
.jpg.webp)
Exocrine cell type
Morphology is what identifies ductal cells. However, there is barely anything to differentiate pancreatic ductal cells from other bodily ductal cells.[6] There is still a lot unknown about these ductal cells. Their molecular identity still needs to be improved; more knowledge is necessary regarding stage-specific markers and the regulators of ductal cell development. It recently was discovered that the ducts start as separate microlumens in a stratified epithelium that expand, attach, and resolve to form the pancreatic ducts. These cells work with intercalating ducts that link to distinct acini and are within the larger ducts in the two core pancreatic ducts (dorsal and ventral duct) that drain into the intestine.[6]
Ductal cells are exocrine, but they are more like endocrine cells when developing. A recent lineage analysis showed that ductal cells came directly from bipotent precursor cells and have the possibility of creating either ductal or endocrine cells. Meanwhile, mature ducts have a restricted ability to transdifferentiate to other types of cells, even when the pancreas is injured.[6]
Ductal cell plasticity
There is disagreement about the plasticity potential of ductal cells in the adult pancreas. In the embryonic pancreas, the endocrine and exocrine cells originate in the pancreatic ducts as progenitor cells.[7] In adult ductal cells, there are observations that these cells take on the identity of progenitor cells when stressed. This proposes the idea that there might be a subgroup of ductal cells that have the ability to dedifferentiate and generate endocrine cells when there is an injury to the pancreas.[7] Essentially, ductal cells function in retaining the adult pancreas β cell mass when injured. However, there is a possibility that this capability is limited to a subtype of ductal cells only, meaning this cannot be a main pathway for pancreas regeneration.[7]
Associated pathologies
Cystic fibrosis
.png.webp)
Cystic fibrosis affects pancreatic ducts as well as many other secretory epithelia. Cystic fibrosis transmembrane conductance regulator (CFTR) is the mutated gene and is essential to chloride and bicarbonate secretion.[3] The abnormal amount of anion secretion causes a reduced amount of ductal water flow. Because of this, the duct's protein concentration increases and causes the duct lumina to get plugged. The onset of cystic fibrosis affects the pancreas more than any other organ (even before birth).[3]
Pancreatitis
The incorrect activation of proteolytic enzymes leads to edema, inflammation, and possible pancreas necrosis, causing acute pancreatitis. The most prominent cause of acute pancreatitis is gallstones.[3] Permanent damage is possible from chronic pancreatitis due to progressive inflammation and the reoccurrence of acute pancreatitis. Acute pancreatitis is caused by mutations in a trypsinogen inhibitor, while a mutation in CFTR causes chronic pancreatitis. In fact, chronic pancreatitis often causes pancreatic adenocarcinoma.[3]
Pancreatic ductal adenocarcinoma

Pancreatic Ductal Adenocarcinoma (PDAC) is one of the most lethal cancers and has an expected survival of five years.[11] Pancreatic adenocarcinoma cells resemble pancreatic ductal cells. Both cell groups show tubule formation, cuboidal shape, and ductal markers. Additionally, acinar and endocrine cells have often been found in many of these cancers, demonstrating plasticity and the possibility that the initial target cells are pancreas progenitor cells.[3] Moreover, human tumors usually go with lower-grade lesions that are called pancreatic intraepithelial neoplasias (PanINs) and are in ducts. Because these lesions are in ducts, this means that it is possible that the beginning target cells are ductal cells.
Breast ductal carcinoma
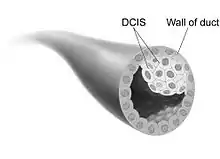
Ductal carcinoma in situ (DCIS) is the proliferation of malignant ductal cells without penetrating the stromal tissue around them.[12] In other words, DCIS is the presence of abnormal cells in a breast's milk duct. It is thought to be the earliest form of breast cancer and is noninvasive (it has not spread from the milk duct and has a low risk of becoming invasive).[13] DCIS can be differentiated into groups based on low, intermediate, and high grade. When comparing the growth potential of normal epithelial cells to DCIS, it is 10 times larger, and the apoptosis rate was also 15 times greater. Furthermore, comedo-type DCIS usually causes necrosis in the duct center and has a more significant threat of reappearance.[12]
The majority of ductal carcinomas are positive for luminal cell markers (CK8, CK18, CK19) but negative for basal cellmarkers (CK5/6 and CK14). In 30% of cases, DCIS is multifocal and usually is in the same breast. There is axillary lymph node invasion in 2-6% of DCIS cases. DCIS is frequently found during mammograms and makes up 25% of screen-detected breast cancers.[12]
The degree of the disease in the breast determines DCIS treatment. In widespread or multifocal DCIS patients, a mastectomy is the recommended choice with the chance of reconstruction.[13] Further clinical trials are being worked on to find an alternative to surgery.
There is not a clear cause for DCIS. This type of cancer comes from genetic mutations in the breast duct cells' DNA. The mutations make the cells look abnormal, but these cells are still not able to leave the breast duct. It is unknown what the exact cause is of this abnormal cell growth that causes DCIS. However, factors that potentially have a role are lifestyle, environment, and passed-down genes.[13]
References
- Reichert M, Rustgi AK (December 2011). "Pancreatic ductal cells in development, regeneration, and neoplasia". The Journal of Clinical Investigation. 121 (12): 4572–4578. doi:10.1172/JCI57131. PMC 3225990. PMID 22133881.
- "English: The pancreas has many functions, served by the endocrine cells in the islets of Langerhans and the exocrine acinar cells. Pancreatic cancer may arise from any of these and disrupt any of their functions". OpenStax College. Retrieved 2022-12-07.
- Grapin-Botton A (March 2005). "Ductal cells of the pancreas". The International Journal of Biochemistry & Cell Biology. 37 (3): 504–510. doi:10.1016/j.biocel.2004.07.010. PMID 15618005.
- Saygin D, Tabib T, Bittar HE, Valenzi E, Sembrat J, Chan SY, et al. (2021-04-21). "Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension". Pulmonary Circulation. 10 (1): 1–15. doi:10.1177/2045894020908782. PMC 7052475. PMID 32166015.
- "cross section: human pancreas". Berkshire Community College Bioscience Image Library. 2019-04-18. Retrieved 2022-12-07.
- MacDonald RJ, Swift GH, Real FX (2010-01-01). Kaestner KH (ed.). "Transcriptional control of acinar development and homeostasis". Progress in Molecular Biology and Translational Science. Development, Differentiation and Disease of the Para-Alimentary Tract. Academic Press. 97: 1–40. doi:10.1016/b978-0-12-385233-5.00001-5. ISBN 9780123852335. PMID 21074728.
- Docherty FM, Russ HA (2019-01-01), "Cell–Cell Interactions Driving Differentiation of Adult Pancreatic Stem Cells", in Reis RL (ed.), Encyclopedia of Tissue Engineering and Regenerative Medicine, Oxford: Academic Press, pp. 367–374, doi:10.1016/b978-0-12-801238-3.65615-5, ISBN 978-0-12-813700-0, retrieved 2022-12-07
- A2-33 (2018-12-19), English: Structure of human cystic fibrosis transmembrane conductance regulator (CFTR) solved by CryoEM, retrieved 2022-12-07
- Patho, English: Tryptic fat tissue necrosis in severe pancreatitis., retrieved 2022-12-07
- Bliss D (2022-10-28). "English: Tranh minh họa ung thư biểu mô ống dẫn sữa tại chỗ (DCIS)". Retrieved 2022-12-07.
- Perusina Lanfranca M, Zhang Y, Girgis A, Kasselman S, Lazarus J, Kryczek I, et al. (April 2020). "Interleukin 22 Signaling Regulates Acinar Cell Plasticity to Promote Pancreatic Tumor Development in Mice". Gastroenterology. 158 (5): 1417–1432.e11. doi:10.1053/j.gastro.2019.12.010. PMC 7197347. PMID 31843590.
- Saygin D, Tabib T, Bittar HE, Valenzi E, Sembrat J, Chan SY, et al. (2010-01-01). Coleman WB, Tsongalis GJ (eds.). "Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension". Pulmonary Circulation. San Diego: Academic Press. 10 (1): 341–349. doi:10.1016/b978-0-12-374418-0.00026-8. ISBN 978-0-12-374418-0. PMC 7052475. PMID 32166015.
- "Ductal carcinoma in situ (DCIS) - Symptoms and causes". Mayo Clinic. Retrieved 2022-12-07.