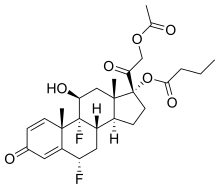
Difluprednate
Difluprednate, sold under the brand name Durezol, is a corticosteroid used for the treatment of post-operative ocular inflammation and pain.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Durezol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609025 |
| License data |
|
| Routes of administration | Eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.636 |
| Chemical and physical data | |
| Formula | C27H34F2O7 |
| Molar mass | 508.559 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It was approved for medical use in the United States in June 2008.[1][2][3] It is available as a generic medication.[4]
Medical uses
Difluprednate is indicated for the treatment of inflammation and pain associated with ocular surgery; and the treatment of endogenous anterior uveitis.[1]
Clinical trials
Difluprednate ophthalmic emulsion 0.05% is also being studied in other ocular inflammatory diseases, including a phase 3 study evaluating difluprednate for the treatment of anterior uveitis[5][6]
References
- "Durezol emulsion". DailyMed. 11 July 2022. Retrieved 7 March 2023.
- "Drug Approval Package: Durezol (Difluprednate) NDA #022212". U.S. Food and Drug Administration (FDA). 25 July 2008. Retrieved 7 March 2023.
- "Sirion Therapeutics Announces FDA Approval of Durezol for Treatment of Postoperative Ocular Inflammation and Pain" (Press release). Sirion Therapeutics, Inc. 2008-06-24. Retrieved 2008-06-30.
- "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- Clinical trial number NCT00501579 for "Study of Difluprednate in the Treatment of Uveitis" at ClinicalTrials.gov
- Sheppard JD, Toyos MM, Kempen JH, Kaur P, Foster CS (May 2014). "Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study". Investigative Ophthalmology & Visual Science. 55 (5): 2993–3002. doi:10.1167/iovs.13-12660. PMC 4581692. PMID 24677110.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.