Bioprospecting
Bioprospecting (also known as biodiversity prospecting) is the exploration of natural sources for small molecules, macromolecules and biochemical and genetic information that could be developed into commercially valuable products for the agricultural,[2][3] aquaculture,[4][5] bioremediation,[4][6] cosmetics,[7][8] nanotechnology,[4][9] or pharmaceutical[2][10] industries. In the pharmaceutical industry, for example, almost one third of all small-molecule drugs approved by the U.S. Food and Drug Administration (FDA) between 1981 and 2014 were either natural products or compounds derived from natural products.[11]
Terrestrial plants, fungi and actinobacteria have been the focus of many past bioprospecting programs,[12] but interest is growing in less explored ecosystems (e.g. seas and oceans) and organisms (e.g. myxobacteria, archaea) as a means of identifying new compounds with novel biological activities.[7][10][13][14] Species may be randomly screened for bioactivity or rationally selected and screened based on ecological, ethnobiological, ethnomedical, historical or genomic information.[10][15][16]
When a region's biological resources or indigenous knowledge are unethically appropriated or commercially exploited without providing fair compensation, this is known as biopiracy.[12][17] Various international treaties have been negotiated to provide countries legal recourse in the event of biopiracy and to offer commercial actors legal certainty for investment. These include the UN Convention on Biological Diversity and the Nagoya Protocol.[2][10]
Other risks associated with bioprospecting are the overharvesting of individual species and environmental damage, but legislation has been developed to combat these also. Examples include national laws such as the US Marine Mammal Protection Act and US Endangered Species Act, and international treaties such as the UN Convention on Biological Diversity, the UN Convention on the Law of the Sea, and the Antarctic Treaty.[10][18]
Bioprospecting-derived resources and products
Agriculture

Bioprospecting-derived resources and products used in agriculture include biofertilizers, biopesticides and veterinary antibiotics. Rhizobium is a genus of soil bacteria used as biofertilizers,[20] Bacillus thuringiensis (also called Bt) and the annonins (obtained from seeds of the plant Annona squamosa) are examples of biopesticides,[21][22][19][23] and valnemulin and tiamulin (discovered and developed from the basidiomycete fungi Omphalina mutila and Clitopilus passeckerianus) are examples of veterinary antibiotics.[24][25]
Bioremediation
Examples of bioprospecting products used in bioremediation include Coriolopsis gallica- and Phanerochaete chrysosporium-derived laccase enzymes, used for treating beer factory wastewater and for dechlorinating and decolorizing paper mill effluent.[9]
Cosmetics and personal care
Cosmetics and personal care products obtained from bioprospecting include Porphyridium cruentum-derived oligosaccharide and oligoelement blends used to treat erythema (rosacea, flushing and dark circles),[7] Xanthobacter autotrophicus-derived zeaxanthin used for skin hydration and UV protection,[8] Clostridium histolyticum-derived collagenases used for skin regeneration,[8] and Microsporum-derived keratinases used for hair removal.[8]
Nanotechnology and biosensors
Because microbial laccases have a broad substrate range, they can be used in biosensor technology to detect a wide range of organic compounds. For example, laccase-containing electrodes are used to detect polyphenolic compounds in wine, and lignins and phenols in wastewater.[9]
Pharmaceuticals
Many of the antibacterial drugs in current clinical use were discovered through bioprospecting including the aminoglycosides, tetracyclines, amphenicols, polymyxins, cephalosporins and other β-lactam antibiotics, macrolides, pleuromutilins, glycopeptides, rifamycins, lincosamides, streptogramins, and phosphonic acid antibiotics.[10][26] The aminoglycoside antibiotic streptomycin, for example, was discovered from the soil bacterium Streptomyces griseus, the fusidane antibiotic fusidic acid was discovered from the soil fungus Acremonium fusidioides, and the pleuromutilin antibiotics (eg. lefamulin) were discovered and developed from the basidiomycete fungi Omphalina mutila and Clitopilus passeckerianus.[10][24]
Other examples of bioprospecting-derived anti-infective drugs include the antifungal drug griseofulvin (discovered from the soil fungus Penicillium griseofulvum),[27] the antifungal and antileishmanial drug amphotericin B (discovered from the soil bacterium Streptomyces nodosus),[28] the antimalarial drug artemisinin (discovered from the plant Artemisia annua),[1][29] and the antihelminthic drug ivermectin (developed from the soil bacterium Streptomyces avermitilis).[30]
Bioprospecting-derived pharmaceuticals have been developed for the treatment of non-communicable diseases and conditions too. These include the anticancer drug bleomycin (obtained from the soil bacterium Streptomyces verticillus),[31] the immunosuppressant drug ciclosporin used to treat autoimmune diseases such as rheumatoid arthritis and psoriasis (obtained from the soil fungus Tolypocladium inflatum),[32] the anti-inflammatory drug colchicine used to treat and prevent gout flares (obtained from the plant Colchicum autumnale),[1] the analgesic drug ziconotide (developed from the cone snail Conus magus),[13] and the acetylcholinesterase inhibitor galantamine used to treat Alzheimer's disease (obtained from plants in the Galanthus genus).[33]
Bioprospecting as a discovery strategy
Bioprospecting has both strengths and weaknesses as a strategy for discovering new genes, molecules, and organisms suitable for development and commercialization.
Strengths
Bioprospecting-derived small molecules (also known as natural products) are more structurally complex than synthetic chemicals, and therefore show greater specificity towards biological targets. This is a big advantage in drug discovery and development, especially pharmacological aspects of drug discovery and development, where off-target effects can cause adverse drug reactions.[10]
Natural products are also more amenable to membrane transport than synthetic compounds. This is advantageous when developing antibacterial drugs, which may need to traverse both an outer membrane and plasma membrane to reach their target.[10]
For some biotechnological innovations to work, it is important to have enzymes that function at unusually high or low temperatures. An example of this is the polymerase chain reaction (PCR), which is dependent on a DNA polymerase that can operate at 60°C and above.[14] In other situations, for example dephosphorylation, it can be desirable to run the reaction at low temperature.[13] Extremophile bioprospecting is an important source of such enzymes, yielding thermostable enzymes such as Taq polymerase (from Thermus aquaticus),[14] and cold-adapted enzymes such as shrimp alkaline phosphatase (from Pandalus borealis).[13]
With the Convention on Biological Diversity (CBD) now ratified by most countries, bioprospecting has the potential to bring biodiversity-rich and technologically advanced nations together, and benefit them both educationally and economically (eg. information sharing, technology transfer, new product development, royalty payment).[2][35]
For useful molecules identified through microbial bioprospecting, scale up of production is feasible at reasonable cost because the producing microorganism can be cultured in a bioreactor.[8][36]
Weaknesses

Although some potentially very useful microorganisms are known to exist in nature (eg. lignocellulose-metabolizing microbes), difficulties have been encountered cultivating these in a laboratory setting.[38] This problem may be resolvable by genetically manipulating easier-to-culture organisms such as Escherichia coli or Streptomyces coelicolor to express the gene cluster responsible for the desired activity.[14][39]
Isolating and identifying the compound(s) responsible for a biological extract's activity can be difficult.[39] Also, subsequent elucidation of the mechanism of action of the isolated compound can be time-consuming.[39] Technological advancements in liquid chromatography, mass spectrometry and other techniques are helping to overcome these challenges.[39]
Implementing and enforcing bioprospecting-related treaties and legislation is not always easy.[2][35] Drug development is an inherently expensive and time-consuming process with low success rates, and this makes it difficult to quantify the value of potential products when drafting bioprospecting agreements.[2] Intellectual property rights may be difficult to award too. For example, legal rights to a medicinal plant may be disputable if it has been discovered by different people in different parts of the world at different times.[2]
Whilst the structural complexity of natural products is generally advantageous in drug discovery, it can make the subsequent manufacture of drug candidates difficult. This problem is sometimes resolvable by identifying the part of the natural product structure responsible for activity and developing a simplified synthetic analogue. This was necessary with the natural product halichondrin B, its simplified analogue eribulin now approved and marketed as an anticancer drug.[40]
Bioprospecting pitfalls
Errors and oversights can occur at different steps in the bioprospecting process including collection of source material, screening source material for bioactivity, testing isolated compounds for toxicity, and identification of mechanism of action.
Collection of source material

Prior to collecting biological material or traditional knowledge, the correct permissions must be obtained from the source country, land owner etc. Failure to do so can result in criminal proceedings and rejection of any subsequent patent applications. It is also important to collect biological material in adequate quantities, to have biological material formally identified, and to deposit a voucher specimen with a repository for long-term preservation and storage. This helps ensure any important discoveries are reproducible.[10][13]
Bioactivity and toxicity testing
When testing extracts and isolated compounds for bioactivity and toxicity, the use of standard protocols (eg. CLSI, ISO, NIH, EURL ECVAM, OECD) is desirable because this improves test result accuracy and reproducibility. Also, if the source material is likely to contain known (previously discovered) active compounds (eg. streptomycin in the case of actinomycetes), then dereplication is necessary to exclude these extracts and compounds from the discovery pipeline as early as possible. In addition, it is important to consider solvent effects on the cells or cell lines being tested, to include reference compounds (ie. pure chemical compounds for which accurate bioactivity and toxicity data are available), to set limits on cell line passage number (eg. 10–20 passages), to include all the necessary positive and negative controls, and to be aware of assay limitations. These steps help ensure assay results are accurate, reproducible and interpreted correctly.[10][13]
Identification of mechanism of action
When attempting to elucidate the mechanism of action of an extract or isolated compound, it is important to use multiple orthogonal assays. Using just a single assay, especially a single in vitro assay, gives a very incomplete picture of an extract or compound's effect on the human body.[41][42] In the case of Valeriana officinalis root extract, for example, the sleep-inducing effects of this extract are due to multiple compounds and mechanisms including interaction with GABA receptors and relaxation of smooth muscle.[41] The mechanism of action of an isolated compound can also be misidentified if a single assay is used because some compounds interfere with assays. For example, the sulfhydryl-scavenging assay used to detect histone acetyltransferase inhibition can give a false positive result if the test compound reacts covalently with cysteines.[42]
Biopiracy
The term biopiracy was coined by Pat Mooney,[43] to describe a practice in which indigenous knowledge of nature, originating with indigenous peoples, is used by others for profit, without authorization or compensation to the indigenous people themselves.[44] For example, when bioprospectors draw on indigenous knowledge of medicinal plants which is later patented by medical companies without recognizing the fact that the knowledge is not new or invented by the patenter, this deprives the indigenous community of their potential rights to the commercial product derived from the technology that they themselves had developed.[45] Critics of this practice, such as Greenpeace,[46] claim these practices contribute to inequality between developing countries rich in biodiversity, and developed countries hosting biotech firms.[45]
In the 1990s many large pharmaceutical and drug discovery companies responded to charges of biopiracy by ceasing work on natural products, turning to combinatorial chemistry to develop novel compounds.[43]
Famous cases of biopiracy

The rosy periwinkle
The rosy periwinkle case dates from the 1950s. The rosy periwinkle, while native to Madagascar, had been widely introduced into other tropical countries around the world well before the discovery of vincristine. Different countries are reported as having acquired different beliefs about the medical properties of the plant.[47] This meant that researchers could obtain local knowledge from one country and plant samples from another. The use of the plant for diabetes was the original stimulus for research. Effectiveness in the treatment of both Hodgkin lymphoma and leukemia were discovered instead.[48] The Hodgkin lymphoma chemotherapeutic drug vinblastine is derivable from the rosy periwinkle.[49]
The Maya ICBG controversy
The Maya ICBG bioprospecting controversy took place in 1999–2000, when the International Cooperative Biodiversity Group led by ethnobiologist Brent Berlin was accused of being engaged in unethical forms of bioprospecting by several NGOs and indigenous organizations. The ICBG aimed to document the biodiversity of Chiapas, Mexico, and the ethnobotanical knowledge of the indigenous Maya people – in order to ascertain whether there were possibilities of developing medical products based on any of the plants used by the indigenous groups.[50][51]
The Maya ICBG case was among the first to draw attention to the problems of distinguishing between benign forms of bioprospecting and unethical biopiracy, and to the difficulties of securing community participation and prior informed consent for would-be bioprospectors.[52]
The neem tree

In 1994, the U.S. Department of Agriculture and W. R. Grace and Company received a European patent on methods of controlling fungal infections in plants using a composition that included extracts from the neem tree (Azadirachta indica), which grows throughout India and Nepal.[53][54][55] In 2000 the patent was successfully opposed by several groups from the EU and India including the EU Green Party, Vandana Shiva, and the International Federation of Organic Agriculture Movements (IFOAM) on the basis that the fungicidal activity of neem extract had long been known in Indian traditional medicine.[55] WR Grace appealed and lost in 2005.[56]
Basmati rice
In 1997, the US corporation RiceTec (a subsidiary of RiceTec AG of Liechtenstein) attempted to patent certain hybrids of basmati rice and semidwarf long-grain rice.[57] The Indian government challenged this patent and, in 2002, fifteen of the patent's twenty claims were invalidated.[58]
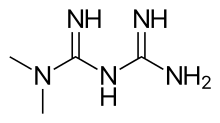
The Enola bean
.jpg.webp)
The Enola bean is a variety of Mexican yellow bean, so called after the wife of the man who patented it in 1999.[59] The allegedly distinguishing feature of the variety is seeds of a specific shade of yellow. The patent-holder subsequently sued a large number of importers of Mexican yellow beans with the following result: "...export sales immediately dropped over 90% among importers that had been selling these beans for years, causing economic damage to more than 22,000 farmers in northern Mexico who depended on sales of this bean."[60] A lawsuit was filed on behalf of the farmers and, in 2005, the US-PTO ruled in favor of the farmers. In 2008, the patent was revoked.[61]
Hoodia gordonii

Hoodia gordonii, a succulent plant, originates from the Kalahari Desert of South Africa. For generations it has been known to the traditionally living San people as an appetite suppressant. In 1996 South Africa's Council for Scientific and Industrial Research began working with companies, including Unilever, to develop dietary supplements based on Hoodia.[62][63][64][65] Originally the San people were not scheduled to receive any benefits from the commercialization of their traditional knowledge, but in 2003 the South African San Council made an agreement with CSIR in which they would receive from 6 to 8% of the revenue from the sale of Hoodia products.[66]
In 2008 after having invested €20 million in R&D on Hoodia as a potential ingredient in dietary supplements for weight loss, Unilever terminated the project because their clinical studies did not show that Hoodia was safe and effective enough to bring to market.[67]
Further cases
The following is a selection of further recent cases of biopiracy. Most of them do not relate to traditional medicines.
- Thirty-six cases of biopiracy in Africa.[68]
- The case of the Maya people's pozol drink.[69][70]
- The case of the Maya and other people's use of Mimosa tenuiflora and many other cases.[71]
- The case of the Andean maca radish.[72]
- The cases of turmeric (India),[73] karela (India), quinoa (Bolivia), oubli berries (Gabon), and others.[74]
- The case of captopril (developed from a Brazilian tribe's arrowhead poison).[75]
Legal and political aspects
Patent law
One common misunderstanding is that pharmaceutical companies patent the plants they collect. While obtaining a patent on a naturally occurring organism as previously known or used is not possible, patents may be taken out on specific chemicals isolated or developed from plants. Often these patents are obtained with a stated and researched use of those chemicals. Generally the existence, structure and synthesis of those compounds is not a part of the indigenous medical knowledge that led researchers to analyze the plant in the first place. As a result, even if the indigenous medical knowledge is taken as prior art, that knowledge does not by itself make the active chemical compound "obvious," which is the standard applied under patent law.
In the United States, patent law can be used to protect "isolated and purified" compounds – even, in one instance, a new chemical element (see USP 3,156,523). In 1873, Louis Pasteur patented a "yeast" which was "free from disease" (patent #141072). Patents covering biological inventions have been treated similarly. In the 1980 case of Diamond v. Chakrabarty, the Supreme Court upheld a patent on a bacterium that had been genetically modified to consume petroleum, reasoning that U.S. law permits patents on "anything under the sun that is made by man." The United States Patent and Trademark Office (USPTO) has observed that "a patent on a gene covers the isolated and purified gene but does not cover the gene as it occurs in nature".[76]
Also possible under US law is patenting a cultivar, a new variety of an existing organism. The patent on the Enola bean (now revoked)[77] was an example of this sort of patent. The intellectual property laws of the US also recognize plant breeders' rights under the Plant Variety Protection Act, 7 U.S.C. §§ 2321–2582.[78]
Convention on Biological Diversity
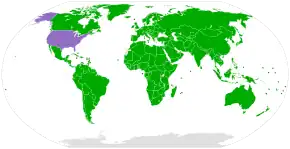
The Convention on Biological Diversity (CBD) came into force in 1993. It secured rights to control access to genetic resources for the countries in which those resources are located. One objective of the CBD is to enable lesser-developed countries to better benefit from their resources and traditional knowledge. Under the rules of the CBD, bioprospectors are required to obtain informed consent to access such resources, and must share any benefits with the biodiversity-rich country.[80] However, some critics believe that the CBD has failed to establish appropriate regulations to prevent biopiracy.[81] Others claim that the main problem is the failure of national governments to pass appropriate laws implementing the provisions of the CBD.[82] The Nagoya Protocol to the CBD, which came into force in 2014, provides further regulations.[83] The CBD has been ratified, acceded or accepted by 196 countries and jurisdictions globally, with exceptions including the Holy See and United States.[79]
Bioprospecting contracts
The requirements for bioprospecting as set by CBD has created a new branch of international patent and trade law, bioprospecting contracts.[2] Bioprospecting contracts lay down the rules of benefit sharing between researchers and countries, and can bring royalties to lesser-developed countries. However, although these contracts are based on prior informed consent and compensation (unlike biopiracy), every owner or carrier of an indigenous knowledge and resources are not always consulted or compensated,[84] as it would be difficult to ensure every individual is included.[85] Because of this, some have proposed that the indigenous or other communities form a type of representative micro-government that would negotiate with researchers to form contracts in such a way that the community benefits from the arrangements.[85] Unethical bioprospecting contracts (as distinct from ethical ones) can be viewed as a new form of biopiracy.[81]
An example of a bioprospecting contract is the agreement between Merck and INBio of Costa Rica.[86]
Traditional knowledge database
Due to previous cases of biopiracy and to prevent further cases, the Government of India has converted traditional Indian medicinal information from ancient manuscripts and other resources into an electronic resource; this resulted in the Traditional Knowledge Digital Library in 2001.[87] The texts are being recorded from Tamil, Sanskrit, Urdu, Persian and Arabic; made available to patent offices in English, German, French, Japanese and Spanish. The aim is to protect India's heritage from being exploited by foreign companies.[88] Hundreds of yoga poses are also kept in the collection.[88] The library has also signed agreements with leading international patent offices such as European Patent Office (EPO), United Kingdom Trademark & Patent Office (UKTPO) and the United States Patent and Trademark Office to protect traditional knowledge from biopiracy as it allows patent examiners at International Patent Offices to access TKDL databases for patent search and examination purposes.[73][89][90]
See also
- Intellectual capital/Intellectual property
- Natural capital
- Biological patent
- Traditional knowledge/Indigenous knowledge
- Pharmacognosy
- Plant breeders' rights
- Bioethics
- Maya ICBG bioprospecting controversy
- International Cooperative Biodiversity Group
- Biological Diversity Act, 2002
- Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) (1994)
- International Treaty on Plant Genetic Resources for Food and Agriculture (2001)
References
- Buenz EJ, Verpoorte R, Bauer BA (January 2018). "The ethnopharmacologic contribution to bioprospecting natural products". Annual Review of Pharmacology and Toxicology. 58 (1): 509–530. doi:10.1146/annurev-pharmtox-010617-052703. PMID 29077533.
- "Mobilizing funding for biodiversity conservation: a user-friendly training guide" (PDF). United Nations. Retrieved 17 July 2020.
- Pandey A, Yarzábal LA (January 2019). "Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments". Applied Microbiology and Biotechnology. 103 (2): 643–657. doi:10.1007/s00253-018-9515-2. PMID 30465306. S2CID 53720063.
- Beattie AJ, Hay M, Magnusson B, de Nys R, Smeathers J, Vincent JF (May 2011). "Ecology and bioprospecting". Austral Ecology. 36 (3): 341–356. doi:10.1111/j.1442-9993.2010.02170.x. PMC 3380369. PMID 22737038.
- Mazarrasa I, Olsen YS, Mayol E, Marbà N, Duarte CM (October 2014). "Global unbalance in seaweed production, research effort and biotechnology markets". Biotechnology Advances. 32 (5): 1028–36. doi:10.1016/j.biotechadv.2014.05.002. PMID 24858315.
- Pascoal F, Magalhães C, Costa R (February 2020). "The link between the ecology of the prokaryotic rare biosphere and its biotechnological potential". Frontiers in Microbiology. 11: Article 231. doi:10.3389/fmicb.2020.00231. PMC 7042395. PMID 32140148.
- Abida H, Ruchaud S, Rios L, Humeau A, Probert I, De Vargas C, Bach S, Bowler C (November 2013). "Bioprospecting marine plankton". Marine Drugs. 11 (11): 4594–4611. doi:10.3390/md11114594. PMC 3853748. PMID 24240981.
- Gupta PL, Rajput M, Oza T, Trivedi U, Sanghvi G (August 2019). "Eminence of microbial products in cosmetic industry". Natural Products and Bioprospecting. 9 (4): 267–278. doi:10.1007/s13659-019-0215-0. PMC 6646485. PMID 31214881.
- Upadhyay P, Shrivastava R, Agrawal PK (June 2016). "Bioprospecting and biotechnological applications of fungal laccase". 3 Biotech. 6 (1): Article 15. doi:10.1007/s13205-015-0316-3. PMC 4703590. PMID 28330085.
- Cushnie TP, Cushnie B, Echeverría J, Fowsantear W, Thammawat S, Dodgson JL, Law S, Clow SM (June 2020). "Bioprospecting for antibacterial drugs: a multidisciplinary perspective on natural product source material, bioassay selection and avoidable pitfalls". Pharmaceutical Research. 37 (7): Article 125. doi:10.1007/s11095-020-02849-1. PMID 32529587. S2CID 219590658.
- Newman DJ, Cragg GM (March 2016). "Natural products as sources of new drugs from 1981 to 2014". Journal of Natural Products. 79 (3): 629–661. doi:10.1021/acs.jnatprod.5b01055. PMID 26852623.
- Cluis C (2013). "Bioprospecting: a new western blockbuster, after the gold rush, the gene rush". The Science Creative Quarterly. No. 8. The Science Creative Quarterly (University of British Columbia). Archived from the original on 2014-04-30. Retrieved 2013-11-04.
- Svenson J (May 2012). "MabCent: Arctic marine bioprospecting in Norway". Phytochemistry Reviews. 12 (3): 567–578. doi:10.1007/s11101-012-9239-3. PMC 3777186. PMID 24078803.
- Sysoev M, Grötzinger SW, Renn D, Eppinger J, Rueping M, Karan R (February 2021). "Bioprospecting of novel extremozymes from prokaryotes—the advent of culture-independent methods". Frontiers in Microbiology. 12: Article 630013. doi:10.3389/fmicb.2021.630013. PMC 7902512. PMID 33643258.
- Saslis-Lagoudakis CH, Savolainen V, Williamson EM, Forest F, Wagstaff SJ, Baral SR, Watson MF, Pendry CA, Hawkins JA (September 2012). "Phylogenies reveal predictive power of traditional medicine in bioprospecting". Proceedings of the National Academy of Sciences of the United States of America. 109 (39): 15835–40. Bibcode:2012PNAS..10915835S. doi:10.1073/pnas.1202242109. PMC 3465383. PMID 22984175.
- Baana K, Angwech H, Malinga GM (May 2018). "Ethnobotanical survey of plants used as repellents against housefly, Musca domestica L. (Diptera: Muscidae) in Budondo Subcounty, Jinja District, Uganda". Journal of Ethnobiology and Ethnomedicine. 14 (1): Article 35. doi:10.1186/s13002-018-0235-6. PMC 5946462. PMID 29747673.
- "Biopiracy". www.merriam-webster.com. Merriam-Webster. 2020. Retrieved 17 July 2020.
- Benson E (February 2012). "Endangered science: the regulation of research by the U.S. Marine Mammal Protection and Endangered Species Acts". Historical Studies in the Natural Sciences. 42 (1): 30–61. doi:10.1525/hsns.2012.42.1.30. PMID 27652415.
- Wani JA, Wali AF, Majid S, Rasool S, Rehman MU, Rashid SM, Ali S, Farooq S, Rasool S, Ahmad A, Qamar W (2020). "Bio-Pesticides: Application and Possible Mechanism of Action". Bioremediation and Biotechnology, Vol 2. pp. 97–119. doi:10.1007/978-3-030-40333-1_6. ISBN 978-3-030-40332-4. S2CID 218939420.
{{cite book}}:|journal=ignored (help)CS1 maint: location missing publisher (link) - John RP, Tyagi RD, Brar SK, Surampalli RY, Prévost D (September 2011). "Bio-encapsulation of microbial cells for targeted agricultural delivery". Critical Reviews in Biotechnology. 31 (3): 211–226. doi:10.3109/07388551.2010.513327. PMID 20879835. S2CID 207467630.
- Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV (2003). "Bacillus thuringiensis crystal proteins that target nematodes". Proceedings of the National Academy of Sciences of the United States of America. 100 (5): 2760–5. Bibcode:2003PNAS..100.2760W. doi:10.1073/pnas.0538072100. PMC 151414. PMID 12598644.
- Gard IE, Gonzalez JM, et al. (September 1992). "Strains of Bacillus thuringiensis insecticidal compositions containing the same US5147640A". Retrieved 2020-07-27.
- Moeschler HF, Pfluger W, Wendisch D (August 1987). "Pure annonin and a process for the preparation thereof US 4689232 A". Retrieved 2020-07-27.
- Kavanagh F, Hervey A, Robbins WJ (September 1951). "Antibiotic substances from basidiomycetes: VIII. Pleurotus multilus (Fr.) Sacc. and Pleurotus passeckerianus Pilat". Proceedings of the National Academy of Sciences of the United States of America. 37 (9): 570–574. Bibcode:1951PNAS...37..570K. doi:10.1073/pnas.37.9.570. PMC 1063423. PMID 16589015.
- de Mattos-Shipley KM, Foster GD, and Bailey AM (June 2017). "Insights into the classical genetics of Clitopilus passeckerianus – the pleuromutilin producing mushroom". Frontiers in Microbiology. 8: Article 1056. doi:10.3389/fmicb.2017.01056. PMC 5465285. PMID 28649239.
- Tilli Tansey; Lois Reynolds, eds. (2000). Post Penicillin Antibiotics: From acceptance to resistance?. Wellcome Witnesses to Contemporary Medicine. History of Modern Biomedicine Research Group. ISBN 978-1-84129-012-6. OL 12568269M. Wikidata Q29581637.
- Beekman AM, Barrow RA (2014). "Fungal metabolites as pharmaceuticals". Australian Journal of Chemistry. 67 (6): 827–843. doi:10.1071/ch13639.
- Procópio RE, Silva IR, Martins MK, Azevedo JL, Araújo JM (2012). "Antibiotics produced by Streptomyces". The Brazilian Journal of Infectious Diseases. 16 (5): 466–71. doi:10.1016/j.bjid.2012.08.014. PMID 22975171.
- Kano S (May 2014). "Artemisinin-based combination therapies and their introduction in Japan". Kansenshogaku Zasshi. 88 (3 Suppl 9–10): 18–25. PMID 24979951.
- Saraiva RG, Dimopoulos G (2020). "Bacterial natural products in the fight against mosquito-transmitted tropical diseases". Natural Product Reports. 37 (3): 338–354. doi:10.1039/c9np00042a. PMID 31544193. S2CID 202731385.
- "Bleomycin". US National Library of Medicine. Retrieved 27 July 2020.
- Borel JF, Kis ZL, Beveridge T (1995). "The History of the Discovery and Development of Cyclosporine (Sandimmune®)". The Search for Anti-Inflammatory Drugs. Boston, MA. pp. 27–63. doi:10.1007/978-1-4615-9846-6_2. ISBN 978-1-4615-9848-0.
{{cite book}}: CS1 maint: location missing publisher (link) - Russo P, Frustaci A, Del Bufalo A, Fini M, Cesario A (2013). "Multitarget drugs of plants origin acting on Alzheimer's disease". Current Medicinal Chemistry. 20 (13): 1686–93. doi:10.2174/0929867311320130008. PMID 23410167.
- Koliou P, Karavasilis V, Theochari M, Pollack SM, Jones RL, Thway K (February 2018). "Advances in the treatment of soft tissue sarcoma: focus on eribulin". Cancer Management and Research. 10: 207–216. doi:10.2147/CMAR.S143019. PMC 5798537. PMID 29440930.
- Sandhu HS. "Bioprospecting: Pros and Cons" (PDF). Punjab Agricultural University. Retrieved 7 July 2021.
- "Pharmaceutical bioreactor / fermentor". American Pharmaceutical Review. Retrieved 7 July 2021.
- Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S, Mir M (2015). "A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone)". ScientificWorldJournal. 2015: Article 816364. doi:10.1155/2015/816364. PMC 4461790. PMID 26106644.
- Buckley M, Wall J. "Microbial energy conversion" (PDF). American Society for Microbiology. Retrieved 7 July 2021.
- Atanasov AG, Zotchev SB, Dirsch VM, INPST, Supuran CT (January 2021). "Natural products in drug discovery: advances and opportunities". Nature Reviews Drug Discovery. 20 (3): 200–216. doi:10.1038/s41573-020-00114-z. PMC 7841765. PMID 33510482.
- "Success story: halichondrin B (NSC 609395) E7389 (NSC 707389)". Developmental Therapeutics Program, National Cancer Institute. Archived from the original on 2009-07-10.
- Houghton PJ, Howes MJ, Lee CC, Steventon G (April 2007). "Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant". Journal of Ethnopharmacology. 110 (3): 391–400. doi:10.1016/j.jep.2007.01.032. PMID 17317057.
- Dahlin JL, Nissink JW, Strasser JM, Francis S, Higgins L, Zhou H, Zhang Z, Walters MA (March 2015). "PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS". Journal of Medicinal Chemistry. 58 (5): 2091–2113. doi:10.1021/jm5019093. PMC 4360378. PMID 25634295.
- Paterson R, Lima N (2016-12-12). Bioprospecting: success, potential and constraints. Paterson, Russell; Lima, Nelson. Cham, Switzerland. ISBN 978-3-319-47935-4. OCLC 965904321.
{{cite book}}: CS1 maint: location missing publisher (link) - Park C, Allaby M. A dictionary of environment and conservation (3 ed.). [Oxford]. ISBN 978-0-19-182632-0. OCLC 970401188.
- Wyatt T (2012). "Biopiracy". Encyclopedia of Transnational Crime & Justice. Thousand Oaks: SAGE Publications, Inc. p. 30. doi:10.4135/9781452218588.n11. ISBN 978-1-4129-9077-6.
- "Agriculture and Food". Green Peace Australia Pacific: What We Do: Food. Greenpeace. Archived from the original on 2008-09-19. Retrieved 2013-11-04.
- "A traditional brew leads to cancer cure". Smithsonian Institution: Migrations in history: Medical Technology. Smithsonian Institution. Archived from the original on 2014-06-21. Retrieved 2013-11-04.
- Hafstein VT (26 July 2004). "The Politics of Origins: Collective Creation Revisited". Journal of American Folklore. 117 (465): 300–315. doi:10.1353/jaf.2004.0073. S2CID 145691975.
- Karasov C (December 2001). "Focus: who reaps the benefits of biodiversity?". Environmental Health Perspectives. 109 (12): A582-7. doi:10.2307/3454734. JSTOR 3454734. PMC 1240518. PMID 11748021.
- Hayden C (2003). When Nature Goes Public: The Making and Unmaking of Bioprospecting in Mexico. Princeton University Press. pp. 100–105. ISBN 978-0-691-09556-1. Retrieved 2013-11-04.
- Feinholz-Klip D, Barrios LG, Lucas JC (2009). "The Limitations of Good Intent: Problems of Representation and Informed Consent in the Maya ICBG Project in Chiapas, Mexico". In Wynberg R, Schroeder D, Chennells R (eds.). Indigenous Peoples, Consent and Benefit Sharing. Springer Netherlands. pp. 315–331. doi:10.1007/978-90-481-3123-5_17. ISBN 978-90-481-3123-5.
- Lavery JV (2007). "Case 1: Community Involvement in Biodiversity Prospecting in Mexico". Ethical Issues in International Biomedical Research: A Casebook. Oxford University Press. pp. 21–43. ISBN 978-0-19-517922-4. Retrieved 2013-11-04.
- "Method for controlling fungi on plants by the aid of a hydrophobic extracted neem oil". google.com. Retrieved 30 April 2018.
- Karen Hoggan for the BBC. May 11, 2000 Neem tree patent revoked Archived 2013-12-26 at the Wayback Machine
- Sheridan C (May 2005). "EPO neem patent revocation revives biopiracy debate". Nature Biotechnology. 23 (5): 511–12. doi:10.1038/nbt0505-511. PMID 15877054. S2CID 29690410.
- BBC News, March 9, 2005 India wins landmark patent battle Archived 2011-06-01 at the Wayback Machine
- "Rice lines bas 867 rt1117 and rt112". google.com. Archived from the original on 30 April 2018. Retrieved 30 April 2018.
- Mukherjee U (June 2008). "A study of the basmati case (India-US basmati rice dispute): The geographical indication perspective". SSRN. doi:10.2139/ssrn.1143209. S2CID 130991379. SSRN 1143209.
- Pallottini L, Garcia E, Kami J, Barcaccia G, Gepts P (1 May 2004). "The Genetic Anatomy of a Patented Yellow Bean". Crop Science. 44 (3): 968–977. doi:10.2135/cropsci2004.0968. Archived from the original on 18 April 2005.
- Goldberg D (2003). "Jack and the Enola Bean". TED Case Studies Number xxx. Danielle Goldberg. Archived from the original on 2013-11-10. Retrieved 2013-11-04.
- "US Patent Office rejects company's claim for bean commonly grown by Latin American farmers". American Association for the Advancement of Science (AAAS). April 2008.
- Maharaj, VJ, Senabe, JV, Horak RM (2008). "Hoodia, a case study at CSIR. Science real and relevant". 2nd CSIR Biennial Conference, CSIR International Convention Centre Pretoria, 17&18 November 2008: 4. hdl:10204/2539.
- Wynberg R, Schroeder D, Chennells R (30 September 2009). Indigenous Peoples, Consent and Benefit Sharing: Lessons from the San-Hoodia Case. Springer. ISBN 978-90-481-3123-5. Retrieved 2013-11-04.
- Vermeylen S (2007). "Contextualizing 'Fair' and 'Equitable': The San's Reflections on the Hoodia Benefit-Sharing Agreement". Local Environment. 12 (4): 423–436. doi:10.1080/13549830701495252. S2CID 153467522.
- Wynberg R (2013-10-13). "Hot air over Hoodia". Grain: Publications: Seedling. Grain. Archived from the original on 2013-11-03. Retrieved 2013-11-03.
- Foster LA (April 2001). "Inventing Hoodia: Vulnerabilities and Epistemic Citizenship in South Africa" (PDF). UCLA Center for the Study of Women: CSW update. UCLA Center for the Study of Women. Archived from the original (PDF) on 2014-04-30. Retrieved 2013-11-04.
- "Nutrition | Unilever". Archived from the original on 2014-04-13. Retrieved 2014-04-10.
- "Africa suffers 36 cases of biopiracy". GhanaWeb. Archived from the original on January 24, 2013. Retrieved 31 March 2006.
- "Biopiracy - a new threat to indigenous rights and culture in Mexico" (PDF). Global Exchange. Archived from the original (PDF) on October 13, 2005. Retrieved 13 October 2005.
- "Biopiracy: the appropriation of indigenous peoples' cultural knowledge" (PDF). New England Law. Archived from the original (PDF) on September 25, 2003. Retrieved 27 February 2008.
- "Of patents & piⓇates". Genetic Resources Action International. Retrieved 18 July 2020.
- "Maca: the dubious aphrodisiac Chinese biopirates took from Peru". Dialogo Chino. 31 October 2019. Retrieved 18 July 2020.
- "Know Instances of Patenting on the UES of Medicinal Plants in India". PIB, Ministry of Environment and Forests. May 6, 2010. Archived from the original on May 10, 2010.
- "The United Kingdom Select Committee on Environmental Audit 1999; Appendices to the Minutes of Evidence, Appendix 7: Trade Related Intellectual Property Rights (TRIPs) and Farmers' Rights". www.parliament.uk. Retrieved 18 July 2020.
- Ellsworth B (December 2010). "Brazil to step up crackdown on "biopiracy"". Ruters. Archived from the original on 7 September 2012. Retrieved 18 July 2020.
- "Department of Commerce: United States Patent and Trademark Office [Docket No. 991027289-0263-02] RIN" (PDF), Federal Register: Notices, Office of the Federal Register of the National Archives and Records Administration, vol. 66, no. 4, pp. 1092–1099, 2001-01-05, archived (PDF) from the original on 2013-02-24, retrieved 2013-11-04
- Crouch D (July 2009). "Mexican yellow bean patent finally cooked". PatentlyO. Retrieved 27 July 2020.
- Chen JM (2006). "The Parable of the Seeds: Interpreting the Plant Variety Protection Act in Furtherance of Innovation Policy". Notre Dame Law Review. 81 (4): 105–166. SSRN 784189.
- "Convention on Biological Diversity: List of parties". United Nations Secretariat of the Convention on Biological Diversity. April 2011. Retrieved 2020-08-03.
- Notman N (August 2012). "Cracking down on wildlife trafficking". Image. Archived from the original on 12 August 2014.
CBD stating that the benefits arising from the use of genetic resources should be shared in a fair and equitable way (Rau, 2010)
- Finegold DL, Bensimon CM, Daar AS, Eaton ML, Godard B, Knoppers BM, Mackie J, Singer PA (July 2005). "Chapter 15: Conclusion: Lessons for Companies and Future Issues". BioIndustry ethics. Elsevier. pp. 331–354. doi:10.1016/b978-012369370-9/50036-7. ISBN 978-0-12-369370-9.
- "Policy Commissions". International Chamber of Commerce: About ICC. International Chamber of Commerce. Archived from the original on 2013-11-02. Retrieved 2013-11-03.
- "The Nagoya Protocol on Access and Benefit-sharing". United Nations Secretariat of the Convention on Biological Diversity. July 2020. Retrieved 2020-08-01.
- Shiva V (2007). "Bioprospecting as Sophisticated Biopiracy". Signs: Journal of Women in Culture and Society. 32 (2): 307–313. doi:10.1086/508502. ISSN 0097-9740. S2CID 144229002.
- Millum J (2010). "How Should the Benefits of Bioprospecting Be Shared?". Hastings Center Report. 40 (1): 24–33. doi:10.1353/hcr.0.0227. ISSN 1552-146X. PMC 4714751. PMID 20169653.
- Eberlee J (2000-01-21). "Assessing the Benefits of Bioprospecting in Latin America" (PDF). IDRC Reports Online. IDRC. Archived from the original (PDF) on 2013-06-23. Retrieved 2013-11-03.
- Bisht TS, Sharma SK, Sati RC, Rao VK, Yadav VK, Dixit AK, Sharma AK, Chopra CS (March 2015). "Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes". Journal of Food Science and Technology. 52 (3): 1543–51. doi:10.1007/s13197-013-1155-z. PMC 4348260. PMID 25745223.
- "India hits back in 'bio-piracy' battle". 2005-12-07. Retrieved 2019-04-11.
- Koshy JP (2010-04-28). "CSIR wing objects to Avesthagen patent claim". Companies. Live Mint. Archived from the original on 2010-04-30. Retrieved 2013-11-04.
- "India Partners with US and UK to Protect Its Traditional Knowledge and Prevent Bio-Piracy" (Press release). Press Information Bureau, Ministry of Health and Family Welfare, Government of India. 2010-04-28. Archived from the original on 2013-05-31. Retrieved 2013-11-04.
Bibliography and resources
- The Secretariat of the Convention on Biological Diversity (United Nations Environment Programme) maintains an information centre which as of April 2006 lists some 3000 "monographs, reports and serials".
- Secretariat of the Convention on Biological Diversity (United Nations Environment Programme), Bibliography of Journal Articles on the Convention on Biological Diversity (March 2006). Contains references to almost 200 articles. Some of these are available in full text from the CBD information centre.
- Shiva V (1997). Biopiracy: The Plunder of Nature and Knowledge. South End Press.
- Chen J (2005). "Biodiversity and Biotechnology: A Misunderstood Relation". Michigan State Law Review. 2005: 51–102. SSRN 782184.
External links
- Out of Africa: Mysteries of Access and Benefit-Sharing – a 2006 report on biopiracy in Africa by The Edmonds Institute
- Cape Town Declaration – Biowatch South Africa
- Genetic Resources Action International (GRAIN)
- Indian scientist denies accusation of biopiracy – SciDev.Net
- African 'biopiracy' debate heats up – SciDev.Net
- Bioprospecting: legitimate research or 'biopiracy'? – SciDev.Net
- ETC Group papers on Biopiracy : Topics include: Monsanto's species-wide patent on all genetically modified soybeans (EP0301749); Synthetic Biology Patents (artificial, unique life forms); Terminator Seed Technology; etc...
- Who Owns Biodiversity, and How Should the Owners Be Compensated?, Plant Physiology, April 2004, Vol. 134, pp. 1295–1307
- Heald PJ (2001). "'Your Friend in the Rain Forest': An Essay on the Rhetoric of Biopiracy". SSRN Electronic Journal. doi:10.2139/ssrn.285177.