Environmental epigenetics
Environmental epigenetics is a branch of epigenetics that studies the influence of external environmental factors on the gene expression of a developing embryo.[1] The way that genes are expressed may be passed down from parent to offspring through epigenetic modifications, although environmental influences do not alter the genome itself.
During embryonic development, epigenetic modifications determine which genes are expressed, which in turn determines the embryo's phenotype. When the offspring is still developing, genes can be turned on and off depending on exposure to certain environmental factors. While certain genes being turned on or off can increase the risk of developmental diseases or abnormal phenotypes, there is also the possibility that the phenotype will be non-functional.[2] Environmental influence on epigenetics is highly variable, but certain environmental factors can greatly increase the risk of detrimental diseases being expressed at both early and adult life stages.[3]
Environmental triggers for epigenetic change
The way that genes are expressed is influenced by the environment that the genome is in. These environmental influences are referred to as triggers and can involve anything that influences normal gene expression. How the genome is expressed depends on the environmental factors present during gestation. It is possible for the environmental effects of epigenetics to be deleterious or to be a natural part of the development pathway.[4] When these environmental factors are detrimental it causes the deactivation of some DNA sequences, which can lead to atypical phenotypes. Some of the most common triggers include diet, temperature, exposure to harmful substances, and lifestyle. These triggers can cause low birth weight, neurological disorders, cancers, autoimmune diseases, and many other malformations.
These epigenetic triggers can change the way that an organism develops and have lifelong effects. Epigenetic changes can be passed down through offspring, present in multiple generations, and continue to change throughout a lifetime. Each sequence of affected DNA is not expressed at the same time. There are specific stages in which these expressions happen during development.[5] The combined epigenetic mechanisms of DNA methylation and histone modification are responsible for how the genome is altered. For example, the suppression of oncogenes is regulated by DNA methylation and whether or not methylation is activated. This activation depends on the environment.[4] When these activations happen, they can be passed down to the next generation through germ-cell differentiation.
Nutrients
Offspring can experience phenotypic changes depending on their access to nutrients. When nutrients are limited during pregnancy, the offspring's phenotypic expression can be disrupted. Nutrient intake is also important during lactation for the purpose of transferring nutrients to the offspring.[6]
Exposures
Exposures to certain triggers such as alcohol or drugs can disrupt the normal expression of the offspring's phenotype. Antipsychotic drugs can lead to abnormal or stunted development during the fetal or embryonic stages.[7]
Stress during pregnancy
When the maternal figure is exposed to stressors it can lead to a greater likelihood of expressing or stunting DNA expression. If the maternal figure experiences high levels of depression or stress it can lead to small litter sizes with lower birth rates. Decreased hormone production is the suspected cause of this.[8]
.jpg.webp)
Temperature
Changes in temperature can have varied effects on an organism. DNA methylation can be impacted by temperature when the temperature deviates from its normal value, preventing regular processes from taking place. Temperature can be considered a stressor in environmental epigenetics since it has the potential to change how offspring respond and react to their environment.[2] Monarch butterflies are an example of how temperature can impact the survival and fitness of an organism.[9] If exposed to stressors such as varying temperatures, these butterflies may express coloring that deviates from their normal color.
Lifestyle choices
Epigenetic marks can result from a number of exposures and choices made by an individual in their lifetime. Exposure to environmental pollutants, psychological stress, dietary choices or restrictions, working habits, and consumption of drugs or alcohol all influence the epigenetics of an individual and what may be passed down to future offspring. [10] Such exposures can affect important processes of epigenetics such as DNA methylation and histone acetylation, influencing the risk for noncommunicable diseases such as obesity.
Multigenerational epigenetic inheritance
Organisms respond to the habitat around them in many different ways, one way is by changing its gene expression to one that is most suitable for their surroundings. More often than not, this has a direct correlation to phenotypic plasticity. Phenotypic plasticity is when a species develops new physical features in response to the environment they’re in. Passing down epigenetics that are in relation to mitotic cell divisions allows for the belief of a possibility that this is also passed down from parents to offspring. Parents could be responsible for the development of new phenotypes in these cases.[11]
Epigenetic inheritance
Epigenetic inheritance refers to passing down or transferring epigenetic information between the parent and offspring. Some believe that these occurrences can be passed down for many generations. For example, the language a given species utilizes develops a specific phenotype that will be passed down from generation to generation.[11]
Cultural inheritance
Cultural inheritance is a behavioral factor that is passed down from generation to generation, similar to inheritance. For example, in rats, mothers that lick and groom their pups pass down a specific behavior to their offspring causing them to do the same to the subsequent generation. Epigenetic inheritance is involved in this, but they are separate things that work together.[11]
Mechanisms influencing epigenetics
DNA replication
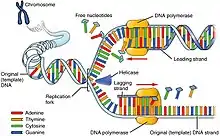
DNA replication is a highly conserved process involving the copying of genetic information from parent one generation to the next. Within this complex process, chromatin disassembles and reassembles in a precise and regulated manner in order to compact large amounts of genetic material into the nucleus, while also maintaining the integrity of epigenetic information carried by histone proteins bound to DNA in the process of cell division. Half of the histones present during replication are from chromatin found in the parent DNA and thus carry the parent's epigenetic information.[12] These epigenetic marks play a critical role in determining chromatin structure and thus gene expression in the newly synthesized DNA. The other half of the histones present in replication are newly synthesized.
DNA methylation
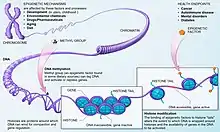
A major formative mechanism of epigenetic modification is DNA methylation. DNA methylation is the process of adding a methyl group to a cytosine base in the DNA strand, via covalent bond. This process is carried out by specific enzymes.[13] These methyl additions can be reversed in a process known as demethylation. The presence or absence of methyl groups can attract proteins involved in gene repression, or inhibit the binding of certain transcription factors, thus preventing methylated genes from being transcribed, ultimately affecting phenotypic expression.[14]
Acetylation
Acetylation is a reaction that introduces an acetyl group into an organic chemical compound, typically by substituting an acetyl group for a hydrogen atom. Deacetylation is the removal of an acetyl group from an organic chemical compound. Histone acetylation and deacetylation affect the three-dimensional structure of chromatin. A more relaxed chromatin structure leads to greater rates of genetic transcription, whereas a tighter structure inhibits transcription.
Transcriptional regulation

Transcriptional regulation is a complex process involving the binding of transcriptional machinery to regulatory proteins—specifically chromatin remodeling or modifying proteins-directly onto a specific target. This may sometimes be facilitated by the contribution of accessory complexes that function primarily to repress and activate transcription in a cell. Transcriptional regulation additionally focuses on the epigenetic regulation of a target locus, as the epigenetic status of the locus determines either the facilitation of or prohibition of transcription. Epigenetic regulation is necessary for the precise deployment of transcriptional programs.[15]
References
- Rosenfeld CS (March 2010). "Animal models to study environmental epigenetics". Biology of Reproduction. 82 (3): 473–488. doi:10.1095/biolreprod.109.080952. PMID 19828779. S2CID 35334680.
- McCaw BA, Stevenson TJ, Lancaster LT (December 2020). "Epigenetic Responses to Temperature and Climate". Integrative and Comparative Biology. 60 (6): 1469–1480. doi:10.1093/icb/icaa049. hdl:2164/16571. PMID 32470117.
- Ho SM, Johnson A, Tarapore P, Janakiram V, Zhang X, Leung YK (2012). "Environmental epigenetics and its implication on disease risk and health outcomes". ILAR Journal. 53 (3–4): 289–305. doi:10.1093/ilar.53.3-4.289. PMC 4021822. PMID 23744968.
- Simmons D (2008). "Epigenetic Influences and Disease | Learn Science at Scitable". Nature Education. 1 (1): 6. Retrieved 2023-04-07.
- Feil R, Fraga MF (January 2012). "Epigenetics and the environment: emerging patterns and implications". Nature Reviews. Genetics. 13 (2): 97–109. doi:10.1038/nrg3142. PMID 22215131. S2CID 21879458.
- LeBaron MJ, Rasoulpour RJ, Klapacz J, Ellis-Hutchings RG, Hollnagel HM, Gollapudi BB (October 2010). "Epigenetics and chemical safety assessment". Mutation Research. 705 (2): 83–95. doi:10.1016/j.mrrev.2010.04.003. PMID 20399890.
- Boyadjieva N, Varadinova M (October 2012). "Epigenetics of psychoactive drugs". The Journal of Pharmacy and Pharmacology. 64 (10): 1349–58. doi:10.1111/j.2042-7158.2012.01475.x. PMID 22943166. S2CID 206003704.
- Barua S, Junaid MA (February 1, 2015). "Lifestyle, pregnancy and epigenetic effects". Epigenomics. 7 (1): 85–102. doi:10.2217/epi.14.71. PMID 25687469.
- Hiyama A, Taira W, Otaki JM (2012). "Color-pattern evolution in response to environmental stress in butterflies". Frontiers in Genetics. 3: 15. doi:10.3389/fgene.2012.00015. PMC 3277265. PMID 22363341.
- Kaushik P, Anderson JT (June 2016). "Obesity: epigenetic aspects". Biomolecular Concepts. 7 (3): 145–155. doi:10.1515/bmc-2016-0010. PMID 27327133. S2CID 207442175.
- Ashe A, Colot V, Oldroyd BP (June 2021). "How does epigenetics influence the course of evolution?". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 376 (1826): 20200111. doi:10.1098/rstb.2020.0111. PMC 8059608. PMID 33866814.
- Budhavarapu VN, Chavez M, Tyler JK (October 2013). "How is epigenetic information maintained through DNA replication?". Epigenetics & Chromatin. 6 (1): 32. doi:10.1186/1756-8935-6-32. PMC 3852060. PMID 24225278.
- Miller RL, Ho SM (March 2008). "Environmental epigenetics and asthma: current concepts and call for studies". American Journal of Respiratory and Critical Care Medicine. 177 (6): 567–573. doi:10.1164/rccm.200710-1511pp. PMC 2267336. PMID 18187692.
- Moore LD, Le T, Fan G (January 2013). "DNA methylation and its basic function". Neuropsychopharmacology. 38 (1): 23–38. doi:10.1038/npp.2012.112. PMC 3521964. PMID 22781841. S2CID 9793643.
- Patmanidi AL, Champeris Tsaniras S, Karamitros D, Kyrousi C, Lygerou Z, Taraviras S (February 2017). "Concise Review: Geminin-A Tale of Two Tails: DNA Replication and Transcriptional/Epigenetic Regulation in Stem Cells". Stem Cells. 35 (2): 299–310. doi:10.1002/stem.2529. PMID 27859962. S2CID 4487813.