Environmental sex determination
Environmental sex determination is the establishment of sex by a non-genetic cue, such as nutrient availability, experienced within a discrete period after fertilization.[1] Environmental factors which often influence sex determination during development or sexual maturation include light intensity and photoperiod, temperature, nutrient availability, and pheromones emitted by surrounding plants or animals. This is in contrast to genotypic sex determination, which establishes sex at fertilization by genetic factors such as sex chromosomes. Under true environmental sex determination, once sex is determined, it is fixed and cannot be switched again. Environmental sex determination is different from some forms of sequential hermaphroditism in which the sex is determined flexibly after fertilization throughout the organism’s life.[2]
_PC301461.JPG.webp)
Adaptive significance
Environmental sex determination is similar to certain forms of sexual selection in that there are oftentimes different and opposing selective pressures on males and females because of the costs of reproduction. Sexual selection is common throughout the tree of life (most known in birds); often resulting in sexual dimorphism, or size and appearance differences between sexes in the same species.[3] In environmental sex determination, selective pressures over evolutionary time have selected for flexibility in sex determination to optimize fitness in a heterogenous environment because of the different costs of sex in males and females.[4] Certain environmental conditions differentially affect each sex such that it would be beneficial to become one sex and not the other.[5] This is especially pertinent for sessile organisms that cannot move to a different environment. In plants, for example, female sexual function is often more energetically expensive because once fertilized they must use significant stored energy to produce fruits, seeds, or sporophytes whereas males must only produce sperm (and sperm-containing structure; antheridium in seedless plants, and pollen in seed plants).
Mechanisms
Lacking genetic information coding for separate sexes such as sex chromosomes, individuals that exhibit environmental sex determination contain genetic information coding for both sexes on autosomes.[6] In general, once exposed to certain environmental cues, epigenetic changes cause developing individuals to become either male or female. Environmental cues that often trigger the development of males or females include temperature, nutrient (or food in the case of animals) and water availability, photoperiod, competitive stress, and pheromones from conspecific individuals.[7][8] Specific mechanisms and cues vary between species.
Taxonomic range
Crustaceans
The amphipod crustacean Gammarus duebeni produces males early in the mating season, and females later, in response to the length of daylight, the photoperiod. Because male fitness improves more than female fitness with increased size, environmental sex determination is adaptive in this system by permitting males to experience a longer growing season than females.[9]
The branchiopod crustacean Daphnia magna parthenogenetically produces male progeny in response to a combination of three environmental factors, namely a reduced photoperiod in autumn, shortage of food and raised population density.[10]
Annelids
Bonellia viridis, a marine worm, has location-dependent sex determination; sex depends on where the larvae land.[11]
Vertebrates

The sex of most amniote vertebrates, such as mammals and birds, is determined genetically.[12] However, some reptiles have temperature-dependent sex determination, where sex is permanently determined by thermal conditions experienced during the middle third of embryonic development.[13][14] The sex of crocodilians and sphenodontians is exclusively determined by temperature. In contrast, squamates (lizards and snakes) and turtles exhibit both genotypic sex determination and temperature-dependent sex determination, although temperature dependence is much more common in turtles than in squamates.[15]
Ferns

Most fern species (with a few exceptions, namely the Salvineales) are homosporous and lack sex chromosomes. Lacking genetic information coding for separate sexes, every fern spore has the capacity to become a male, female, or hermaphroditic gametophyte depending on the environment.[16][17] In many fern species, including Ceratopteris richardii, environmental sex determination is linked to breeding systems.[18] Fern gametophytes exhibit a wide variety of breed systems which can be divided into outcrossing and inbreeding. To promote outcrossing, female gametophytes release a chemical pheromone known as Antheridiogen which controls the sex of nearby developing gametophytes.[19] Antheridiogen secreted by females promotes the development of nearby asexual gametophytes into males. This is adaptive because inducing maleness increases the probability of outcrossing as males provide sperm for the females rather than the females becoming hermaphroditic (or bisexual) and self-fertilizing. However, if no fertilization occurs, the female gametophyte can still become hermaphroditic and self-fertilize if the conditions are conducive to growth, ultimately resulting in inbreeding depression.
Additionally, similar to crocodilians, homosporous fern gametophyte sex is determined by the abiotic environment in accordance with the size-advantage model. In stressful environments (crowding or nutrient stress), gametophytes are smaller and develop into males. While in more favorable growing conditions, gametophytes are larger and develop into females.[20][21]
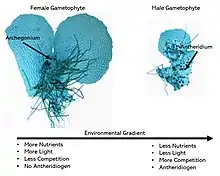
Moss

Moss gametophytes can be either asexual, female, male, or hermaphroditic like ferns. Unlike homosporous ferns, moss gametophytes can be either monoicous or dioicous (similar to monoecious and dioecious in vascular plants), with most studied dioicous species exhibiting genetic sex determination via the UV sex chromosome sex determination system. Some monoicous moss species such as Splachnum ampullaceum exhibit environmental sex determination during early development, with low light, low pH, and low nutrient availability all promoting male development.[22] In the presence of auxin, a widespread plant hormone, or gibberellins, compounds similar to Antheridiogen in ferns, both female and male individuals invest more in sexual structures (antheridia and archegonia).[23] Environmental sex determination in moss is fundamentally different from the spatial segregation of sexes, the occurrence of environmentally mediated sex ratios in moss patches, observed in sexually static moss species. Spatial segregation of the sexes in mosses is caused by differential survival rates between sexes as a result of the competitive advantage of female moss.[24][25] This leads to female dominated populations maintained by asexual reproduction and minimal sexual reproduction. In contrast, environmental sex determination is the dynamic development of females or males in different environmental conditions.
Angiosperms
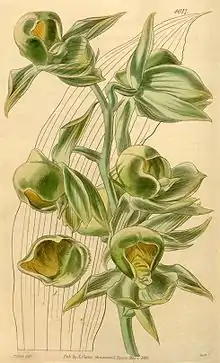
Many angiosperms exhibit sequential hermaphroditism, meaning that they can switch sexes continually throughout their life based on the current conditions and resource availability to optimize fitness each flowering season.[26] But sequential hermaphroditism and environmental sex determination are not mutually exclusive. For example, Catasetum viridiflavum, an epiphyte (plant that grows on another plant) in the Orchidaceae family exhibits sequential hermaphroditism where the younger, smaller individuals have male inflorescences and the older, larger individuals have female inflorescences, but sex expression is also strongly influenced by light intensity. Individuals in high light are more often female and individuals in the low light are more often male, regardless of size.[27][28] In higher light, individuals produce more ethylene, a common plant hormone, which promotes the formation of female flowers.
References
- JANZEN, F. J.; PHILLIPS, P. C. (November 2006). "Exploring the evolution of environmental sex determination, especially in reptiles". Journal of Evolutionary Biology. 19 (6): 1775–1784. doi:10.1111/j.1420-9101.2006.01138.x. ISSN 1010-061X. PMID 17040374. S2CID 15485510.
- Warner, Robert R. (January 1975). "The Adaptive Significance of Sequential Hermaphroditism in Animals". The American Naturalist. 109 (965): 61–82. doi:10.1086/282974. ISSN 0003-0147. S2CID 84279130.
- Parker, G.A. (1979), "Sexual Selection and Sexual Conflict", Sexual Selection and Reproductive Competition in Insects, Elsevier, pp. 123–166, doi:10.1016/b978-0-12-108750-0.50010-0, ISBN 978-0-12-108750-0, retrieved 2020-11-17
- DeSoto, Lucía; Quintanilla, Luis G.; Méndez, Marcos (November 2008). "Environmental sex determination in ferns: effects of nutrient availability and individual density inWoodwardia radicans". Journal of Ecology. 96 (6): 1319–1327. doi:10.1111/j.1365-2745.2008.01425.x. ISSN 0022-0477.
- McCabe, J.; Dunn, A. M. (1997). "Adaptive significance of environmental sex determination in an amphipod". Journal of Evolutionary Biology. 10 (4): 515. doi:10.1007/s000360050039. ISSN 1010-061X.
- Bull, J. J.; Vogt, R. C.; Bulmer, M. G. (March 1982). "Heritability of Sex Ratio in Turtles with Environmental Sex Determination". Evolution. 36 (2): 333–341. doi:10.2307/2408052. ISSN 0014-3820. JSTOR 2408052. PMID 28563174.
- Adams, Jonathan; Greenwood, Paul; Naylor, Caroline (March 1987). "Evolutionary Aspects of Environmental Sex Determination". International Journal of Invertebrate Reproduction and Development. 11 (2): 123–135. doi:10.1080/01688170.1987.10510273. ISSN 0168-8170.
- Dunn, Alison M.; Hogg, John C.; Kelly, Andrew; Hatcher, Melanie J. (January 2005). "Two cues for sex determination in Gammarus duebeni : Adaptive variation in environmental sex determination?". Limnology and Oceanography. 50 (1): 346–353. Bibcode:2005LimOc..50..346D. doi:10.4319/lo.2005.50.1.0346. ISSN 0024-3590.
- McCabe, J.; Dunn, A. M. (1997). "Adaptive significance of environmental sex determination in an amphipod". Journal of Evolutionary Biology. 10 (4): 515–527. doi:10.1046/j.1420-9101.1997.10040515.x.
- Kato, Yasuhiko; Kobayashi, Kaoru; Watanabe, Hajime; Iguchi, Taisen (2011). "Environmental Sex Determination in the Branchiopod Crustacean Daphnia magna: Deep Conservation of a Doublesex Gene in the Sex-Determining Pathway". PLOS Genetics. 7 (3): 1–12. doi:10.1371/journal.pgen.1001345. PMC 3063754. PMID 21455482.
- Gilbert, Scott (2006). Developmental biology (8th ed.). Sunderland, Mass.: Sinauer Associates. p. 552. ISBN 9780878932504.
- James J. Bull (1983). Evolution of Sex Determining Mechanisms. Menlo Park, California: Benjamin Cummings. ISBN 978-0-201-11242-9.
- Fredric J. Janzen; Gary L. Paukstis (1991). "Environmental sex determination in reptiles: ecology, evolution, and experimental design". Quarterly Review of Biology. 66 (2): 149–179. doi:10.1086/417143. JSTOR 2830229. PMID 1891591. S2CID 35956765.
- Nicole Valenzuela; Valentine A. Lance, eds. (2004). Temperature Dependent Sex Determination in Vertebrates. Smithsonian Institution. ISBN 978-1-58834-203-4.
- Janzen, Fredric J.; Krenz, James G. (2004). "Phylogenetics: which was first, TSD or GSD?". In Nicole Valenzuela; Valentine A. Lance (eds.). Temperature Dependent Sex Determination in Vertebrates (PDF). Smithsonian Institution. pp. 121–130. ISBN 978-1-58834-203-4.
- Goodnoe, Taylor T.; Hill, Jeffrey P.; Aho, Ken (April 2016). "Effects of variation in carbon, nitrogen, and phosphorus molarity and stoichiometry on sex determination in the fern Ceratopteris richardii". Botany. 94 (4): 249–259. doi:10.1139/cjb-2015-0187. ISSN 1916-2790.
- DeSoto, Lucía; Quintanilla, Luis G.; Méndez, Marcos (November 2008). "Environmental sex determination in ferns: effects of nutrient availability and individual density inWoodwardia radicans". Journal of Ecology. 96 (6): 1319–1327. doi:10.1111/j.1365-2745.2008.01425.x. ISSN 0022-0477.
- Goodnoe, Taylor T.; Hill, Jeffrey P. (2018-05-20). "Plasticity of female reproductive resource allocation depends on the presence or absence of prior environmental sex determination inCeratopteris richardii". Ecology and Evolution. 8 (12): 6133–6143. doi:10.1002/ece3.4159. ISSN 2045-7758. PMC 6024121. PMID 29988448.
- Chiou, Wen-Liang; Farrar, Donald R. (May 1997). "Antheridiogen production and response in Polypodiaceae species". American Journal of Botany. 84 (5): 633–640. doi:10.2307/2445900. ISSN 0002-9122. JSTOR 2445900. PMID 21708616.
- KORPELAINEN, HELENA (April 1994). "Growth, sex determination and reproduction of Dryopteris filix-mas (L.) Schott gametophytes under varying nutritional conditions". Botanical Journal of the Linnean Society. 114 (4): 357–366. doi:10.1111/j.1095-8339.1994.tb01840.x. ISSN 0024-4074.
- Pérez-Escobar, Oscar Alejandro; Chomicki, Guillaume; Condamine, Fabien L.; de Vos, Jurriaan M.; Martins, Aline C.; Smidt, Eric C.; Klitgård, Bente; Gerlach, Günter; Heinrichs, Jochen (2017-10-10). "Multiple Geographical Origins of Environmental Sex Determination enhanced the diversification of Darwin's Favourite Orchids". Scientific Reports. 7 (1): 12878. Bibcode:2017NatSR...712878P. doi:10.1038/s41598-017-12300-y. ISSN 2045-2322. PMC 5635016. PMID 29018291.
- Cameron, Randall G.; Wyatt, Robert (1990). "Spatial Patterns and Sex Ratios in Dioecious and Monoecious Mosses of the Genus Splachnum". The Bryologist. 93 (2): 161. doi:10.2307/3243620. ISSN 0007-2745. JSTOR 3243620.
- BHATLA, S. C.; CHOPRA, R. N. (1981). "Hormonal Regulation of Gametangial Formation in the MossBryum argenteumHedw". Journal of Experimental Botany. 32 (6): 1243–1256. doi:10.1093/jxb/32.6.1243. ISSN 0022-0957.
- Bowker, Matthew A.; Stark, Lloyd R.; McLetchie, D. Nicholas; Mishler, Brent D. (April 2000). "Sex expression, skewed sex ratios, and microhabitat distribution in the dioecious desert moss Syntrichia caninervis (Pottiaceae)". American Journal of Botany. 87 (4): 517–526. doi:10.2307/2656595. ISSN 0002-9122. JSTOR 2656595. PMID 10766723.
- Kobayashi, Kazuya; Hasegawa, Eisuke (April 2016). "A female-biased sex ratio reduces the twofold cost of sex". Scientific Reports. 6 (1): 23982. Bibcode:2016NatSR...623982K. doi:10.1038/srep23982. ISSN 2045-2322. PMC 4817508. PMID 27035400.
- Policansky, D (November 1982). "Sex Change in Plants and Animals". Annual Review of Ecology and Systematics. 13 (1): 471–495. doi:10.1146/annurev.es.13.110182.002351. ISSN 0066-4162.
- Pérez-Escobar, Oscar Alejandro; Chomicki, Guillaume; Condamine, Fabien L.; de Vos, Jurriaan M.; Martins, Aline C.; Smidt, Eric C.; Klitgård, Bente; Gerlach, Günter; Heinrichs, Jochen (2017-10-10). "Multiple Geographical Origins of Environmental Sex Determination enhanced the diversification of Darwin's Favourite Orchids". Scientific Reports. 7 (1): 12878. Bibcode:2017NatSR...712878P. doi:10.1038/s41598-017-12300-y. ISSN 2045-2322. PMC 5635016. PMID 29018291.
- Zimmerman, Jess K. (April 1991). "Ecological Correlates of Labile Sex Expression in the Orchid Catasetum Viridiflavum". Ecology. 72 (2): 597–608. doi:10.2307/2937200. ISSN 0012-9658. JSTOR 2937200.