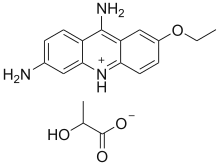
Ethacridine lactate
Ethacridine lactate (ethacridine monolactate monohydrate, acrinol, trade name Rivanol) is an aromatic organic compound based on acridine. Its formal name is 2-ethoxy-6,9-diaminoacridine monolactate monohydrate. It forms orange-yellow crystals with a melting point of 226 °C and it has a stinging smell.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.015.826 |
| Chemical and physical data | |
| Formula | C18H21N3O4 |
| Molar mass | 343.37 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Its primary use is as an antiseptic[1] in solutions of 0.1%. It is effective against mostly Gram-positive bacteria, such as Streptococci and Staphylococci, but ineffective against Gram-negative bacteria such as Pseudomonas aeruginosa.[2]
Ethacridine is also used as an agent for second trimester abortion. Up to 150 ml of a 0.1% solution is instilled extra-amniotically using a foley catheter. After 20 to 40 hours, 'mini labor' ensues. In China, an intra-amniotic method has also been used.[3] Ethacridine as an abortifacient is found to be safer and better tolerated than 20% hypertonic saline.
References
- Merck Index, 11th Ed., 3668
- Adarchenko AA, Krasil'nikov AP, Sobeshchuk OP (1989). "[Antiseptic sensitivity of clinical strains of Pseudomonas aeruginosa]". Antibiotiki I Khimioterapiia (in Russian). 34 (12): 902–7. PMID 2517004.
- Tien, K. H. (1983). "Intraamniotic injection of ethacridine for second-trimester induction of labor". Obstetrics and Gynecology. 61 (6): 733–736. PMID 6843933.