endo-exo isomerism
In organic chemistry, endo–exo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system.[1] The prefix endo is reserved for the isomer with the substituent located closest, or "syn", to the longest bridge. The prefix exo is reserved for the isomer with the substituent located farthest, or "anti", to the longest bridge. Here "longest" and "shortest" refer to the number of atoms that comprise the bridge. This type of molecular geometry is found in norbornane systems such as dicyclopentadiene.
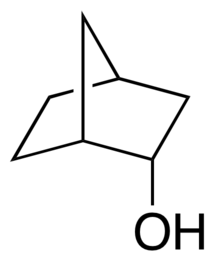
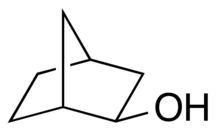
Comparison of endo-norborneol (left) and exo-norborneol
The terms endo and exo are used in a similar sense in discussions of the stereoselectivity in Diels–Alder reactions.
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "endo, exo, syn, anti". doi:10.1351/goldbook.E02094
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.