Fast neutron therapy
Fast neutron therapy utilizes high energy neutrons typically between 50 and 70 MeV to treat cancer. Most fast neutron therapy beams are produced by reactors, cyclotrons (d+Be) and linear accelerators. Neutron therapy is currently available in Germany, Russia, South Africa and the United States. In the United States, one treatment center is operational, in Seattle, Washington. The Seattle center uses a cyclotron which produces a proton beam impinging upon a beryllium target.
| Fast neutron therapy | |
|---|---|
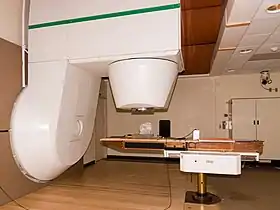 Patient treating room for neutron radiation therapy | |
| ICD-10-PCS | D?0?5ZZ |
| ICD-9 | 92.26 |
Advantages
Radiation therapy kills cancer cells in two ways depending on the effective energy of the radiative source. The amount of energy deposited as the particles traverse a section of tissue is referred to as the linear energy transfer (LET). X-rays produce low LET radiation, and protons and neutrons produce high LET radiation. Low LET radiation damages cells predominantly through the generation of reactive oxygen species, see free radicals. The neutron is uncharged and damages cells by direct effect on nuclear structures. Malignant tumors tend to have low oxygen levels and thus can be resistant to low LET radiation. This gives an advantage to neutrons in certain situations. One advantage is a generally shorter treatment cycle. To kill the same number of cancerous cells, neutrons require one third the effective dose as protons.[1] Another advantage is the established ability of neutrons to better treat some cancers, such as salivary gland, adenoid cystic carcinomas and certain types of brain tumors, especially high-grade gliomas [2]
LET
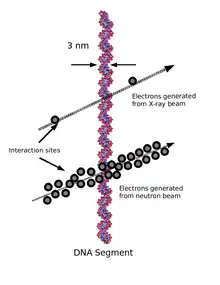
When therapeutic energy X-rays (1 to 25 MeV) interact with cells in human tissue, they do so mainly by Compton interactions, and produce relatively high energy secondary electrons. These high energy electrons deposit their energy at about 1 keV/µm.[3] By comparison, the charged particles produced at a site of a neutron interaction may deliver their energy at a rate of 30–80 keV/µm. The amount of energy deposited as the particles traverse a section of tissue is referred to as the linear energy transfer (LET). X-rays produce low LET radiation, and neutrons produce high LET radiation.
Because the electrons produced from X-rays have high energy and low LET, when they interact with a cell typically only a few ionizations will occur. It is likely then that the low LET radiation will cause only single strand breaks of the DNA helix. Single strand breaks of DNA molecules can be readily repaired, and so the effect on the target cell is not necessarily lethal. By contrast, the high LET charged particles produced from neutron irradiation cause many ionizations as they traverse a cell, and so double-strand breaks of the DNA molecule are possible. DNA repair of double-strand breaks are much more difficult for a cell to repair, and more likely to lead to cell death.
DNA repair mechanisms are quite efficient,[4] and during a cell's lifetime many thousands of single strand DNA breaks will be repaired. A sufficient dose of ionizing radiation, however, delivers so many DNA breaks that it overwhelms the capability of the cellular mechanisms to cope.
Heavy ion therapy (e.g. carbon ions) makes use of the similarly high LET of 12C6+ ions.[5][6]
Because of the high LET, the relative radiation damage (relative biological effect or RBE) of fast neutrons is 4 times that of X-rays,[7][8] meaning 1 rad of fast neutrons is equal to 4 rads of X-rays. The RBE of neutrons is also energy dependent, so neutron beams produced with different energy spectra at different facilities will have different RBE values.
Oxygen effect
The presence of oxygen in a cell acts as a radiosensitizer, making the effects of the radiation more damaging. Tumor cells typically have a lower oxygen content than normal tissue. This medical condition is known as tumor hypoxia and therefore the oxygen effect acts to decrease the sensitivity of tumor tissue.[9] The oxygen effect may be quantitatively described by the Oxygen Enhancement Ratio (OER). Generally it is believed that neutron irradiation overcomes the effect of tumor hypoxia,[10] although there are counterarguments. [11]
Clinical uses
The efficacy of neutron beams for use on prostate cancer has been shown through randomized trials.[12][13][14] Fast neutron therapy has been applied successfully against salivary gland tumors.[15][16][17][18][19][20][21][22] Adenoid cystic carcinomas have also been treated.[23][24] Various other head and neck tumors have been examined.[25][26][27]
Side effects
No cancer therapy is without the risk of side effects. Neutron therapy is a very powerful nuclear scalpel that has to be utilized with exquisite care. For instance, some of the most remarkable cures it has been able to achieve are with cancers of the head and neck. Many of these cancers cannot effectively be treated with other therapies. However, neutron damage to nearby vulnerable areas such as the brain and sensory neurons can produce irreversible brain atrophy, blindness, etc. The risk of these side effects can be greatly mitigated by several techniques, but they cannot be totally eliminated. Moreover, some patients are more susceptible to such side effects than others and this cannot be predicted. The patient ultimately must decide whether the advantages of a possibly lasting cure outweigh the risks of this treatment when faced with an otherwise incurable cancer.[28]
Fast neutron centers
Several centers around the world have used fast neutrons for treating cancer. Due to lack of funding and support, at present only three are active in the USA. The University of Washington and the Gershenson Radiation Oncology Center operate fast neutron therapy beams and both are equipped with a Multi-Leaf Collimator (MLC) to shape the neutron beam.[29][30][31]
University of Washington
The Radiation Oncology Department[32] operates a proton cyclotron that produces fast neutrons from directing 50.5 MeV protons onto a beryllium target. The UW Cyclotron is equipped with a gantry mounted delivery system an MLC to produce shaped fields. The UW Neutron system is referred to as the Clinical Neutron Therapy System (CNTS).[33] The CNTS is typical of most neutron therapy systems. A large, well shielded building is required to cut down on radiation exposure to the general public and to house the necessary equipment.
 UW Cyclotron
UW Cyclotron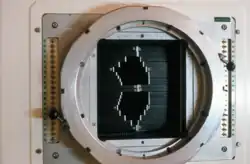 Multi-Leaf Collimator (MLC) used to shape the neutron beam
Multi-Leaf Collimator (MLC) used to shape the neutron beam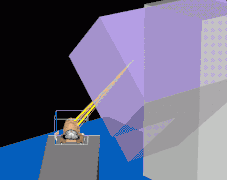 Schematic of a treatment field delivery. The patient couch has been rotated, along with the gantry so the neutron beam will enter obliquely, to give maximum sparing of normal tissue.
Schematic of a treatment field delivery. The patient couch has been rotated, along with the gantry so the neutron beam will enter obliquely, to give maximum sparing of normal tissue.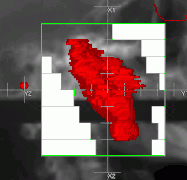 Example of a treatment neutron field collimated using a neutron MLC
Example of a treatment neutron field collimated using a neutron MLC
A beamline transports the proton beam from the cyclotron to a gantry system. The gantry system contains magnets for deflecting and focusing the proton beam onto the beryllium target. The end of the gantry system is referred to as the head, and contains dosimetry systems to measure the dose, along with the MLC and other beam shaping devices. The advantage of having a beam transport and gantry are that the cyclotron can remain stationary, and the radiation source can be rotated around the patient. Along with varying the orientation of the treatment couch which the patient is positioned on, variation of the gantry position allows radiation to be directed from virtually any angle, allowing sparing of normal tissue and maximum radiation dose to the tumor.
During treatment, only the patient remains inside the treatment room (called a vault) and the therapists will remotely control the treatment, viewing the patient via video cameras. Each delivery of a set neutron beam geometry is referred to as a treatment field or beam. The treatment delivery is planned to deliver the radiation as effectively as possible, and usually results in fields that conform to the shape of the gross target, with any extension to cover microscopic disease.
Karmanos Cancer Center / Wayne State University
The neutron therapy facility at the Gershenson Radiation Oncology Center at Karmanos Cancer Center/Wayne State University (KCC/WSU) in Detroit bears some similarities to the CNTS at the University of Washington, but also has many unique characteristics. This unit was decommissioned in 2011.
 Schematic of MLC
Schematic of MLC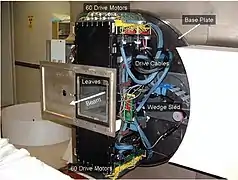 Photo of the MLC
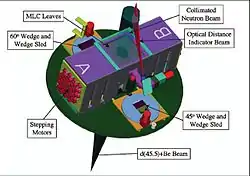
Photo of the MLC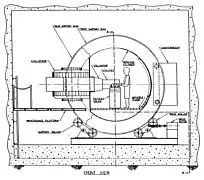 Schematic of the KCC/WSU gantry mounted superconducting cyclotron
Schematic of the KCC/WSU gantry mounted superconducting cyclotron
While the CNTS accelerates protons, the KCC facility produces its neutron beam by accelerating 48.5 MeV deuterons onto a beryllium target. This method produces a neutron beam with depth dose characteristics roughly similar to those of a 4 MV photon beam. The deuterons are accelerated using a gantry mounted superconducting cyclotron (GMSCC), eliminating the need for extra beam steering magnets and allowing the neutron source to rotate a full 360° around the patient couch.
The KCC facility is also equipped with an MLC beam shaping device,[34] the only other neutron therapy center in the USA besides the CNTS. The MLC at the KCC facility has been supplemented with treatment planning software that allows for the implementation of Intensity Modulated Neutron Radiotherapy (IMNRT), a recent advance in neutron beam therapy which allows for more radiation dose to the targeted tumor site than 3-D neutron therapy.[35]
KCC/WSU has more experience than anyone in the world using neutron therapy for prostate cancer, having treated nearly 1,000 patients during the past 10 years.
Fermilab / Northern Illinois University
The Fermilab neutron therapy center first treated patients in 1976,[36] and since that time has treated over 3,000 patients. In 2004, the Northern Illinois University began managing the center. The neutrons produced by the linear accelerator at Fermilab have the highest energies available in the US and among the highest in the world [37][38][39]
The Fermilab center was decommissioned in 2013.[40]
See also
References
- Keyhandokht Shahri, Laleh Motavalli, and Hashem Hakimabad."Neutron Applications in Cancer Treatment" Hellenic Journal of Nuclear Medicine 14:2(May–August 2011)
- Feng-Yi Yang, Wen-Yuan Chang, Jia-Je Li, Hsin-Ell Wang, Jyh-Cheng Chen, and Chi-Wei Chang."Pharmacokinetic Analysis and Uptake of 18F-FBPA-Fr After Ultrasound-Induced Blood–Brain Barrier Disruption for Potential Enhancement of Boron Delivery for Neutron Capture Therapy" Journal of Nuclear Medicine 55:616–621(2014)
- Johns HE and Cunningham JR. The Physics of Radiology. Charles C Thomas 3rd edition 1978
- Goodsell DS. Fundamentals of Cancer Medicine The Molecular Perspective: Double-Stranded DNA Breaks The Oncologist, Vol. 10, No. 5, 361–362, May 2005
- Kubota N, Suzuki M, Furusawa Y, Ando K, Koike S, Kanai T, Yatagai F, Ohmura M, Tatsuzaki H, Matsubara S, et al. A comparison of biological effects of modulated carbon-ions and fast neutrons in human osteosarcoma cells. International Journal of Radiation Oncology, Biology, Physics, Volume 33, Issue 1, 30 August 1995, Pages 135–141
- German Cancer Research Center
- Pignol JP, Slabbert J and Binns P. Monte Carlo simulation of fast neutron spectra: Mean lineal energy estimation with an effectiveness function and correlation to RBE. International Journal of Radiation Oncology, Biology, Physics, Volume 49, Issue 1, 1 January 2001, Pages 251–260
- Theron T, Slabbert J, Serafin A and Böhm L. The merits of cell kinetic parameters for the assessment of intrinsic cellular radiosensitivity to photon and high linear energy transfer neutron irradiation. International Journal of Radiation Oncology, Biology, Physics, Volume 37, Issue 2, 15 January 1997, Pages 423–428
- Vaupel P, Harrison L. Tumor Hypoxia: Causative Factors, Compensatory Mechanisms, and Cellular Response The Oncologist 2004;9(suppl 5):4–9
- Wambersie A, Richard F, Breteau N. Development of fast neutron therapy worldwide. Radiobiological, clinical and technical aspects. Acta Oncol. 1994;33(3):261-74.
- Warenius HM, White R, Peacock JH, Hanson J, Richard A. Britten, Murray D. The Influence of Hypoxia on the Relative Sensitivity of Human Tumor Cells to 62.5 MeV (p→Be) Fast Neutrons and 4 MeV Photons. Radiation Research 154, 54–63 (2000)
- Russell KJ, Caplan RJ, Laramore GE, et al. Photon versus fast neutron external beam radiotherapy in the treatment of locally advanced prostate cancer: results of a randomized prospective trial. International Journal of Radiation Oncology, Biology, Physics 28(1): 47–54, 1993.
- Haraf DJ, Rubin SJ, Sweeney P, Kuchnir FT, Sutton HG, Chodak GW and Weichselbaum RR. Photon neutron mixed-beam radiotherapy of locally advanced prostate cancer. International Journal of Radiation Oncology, Biology, Physics, Volume 33, Issue 1, 30 August 1995, Pages 3–14
- Forman J, Ben-Josef E, Bolton SE, Prokop S and Tekyi-Mensah S . A randomized prospective trial of sequential neutron-photon vs. photon-neutron irradiation in organ confined prostate cancer. International Journal of Radiation Oncology, Biology, Physics, Volume 54, Issue 2, Supplement 1, 1 October 2002, Pages 10–11
- Douglas JD, Koh WJ, Austin-Seymour, M, Laramore GE. Treatment of Salivary Gland Neoplasms with fast neutron Radiotherapy. Arch Otolaryngol Head Neck Surg Vol 129 944–948 Sep 2003
- Laramore GE, Krall JM, Griffin TW, Duncan W, Richter MP, Saroja KR, Maor MH, Davis LW. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Int J Radiat Oncol Biol Phys. 1993 Sep 30;27(2):235-40.
- Laramore GE. Fast neutron radiotherapy for inoperable salivary gland tumors: is it the treatment of choice?Int J Radiat Oncol Biol Phys. 1987 Sep;13(9):1421-3.
- Prott FJ, Micke O, Pötter R, Haverkamp U, Schüller P and Willich N. 2137 Results of fast neutron therapy of adenoid cystic carcinoma of the salivary glands. International Journal of Radiation Oncology, Biology, Physics, Volume 39, Issue 2, Supplement 1, 1997, Page 309
- Saroja KR, Mansell J, Hendrickson FR, et al.: An update on malignant salivary gland tumors treated with neutrons at Fermilab. Int J Radiat Oncol Biol Phys 13 (9): 1319–25, 1987.
- Buchholz TA, Laramore GE, Griffin BR, et al.: The role of fast neutron radiation therapy in the management of advanced salivary gland malignant neoplasms. Cancer 69 (11): 2779–88, 1992.
- Krüll A, Schwarz R, Engenhart R, et al.: European results in neutron therapy of malignant salivary gland tumors. Bull Cancer Radiother 83 (Suppl): 125-9s, 1996
- See also the NCI Salivary Cancer Page
- Adenoid Cystic Carcinoma Neutron Radiation Therapy Archived September 25, 2006, at the Wayback Machine
- Douglas JG, Laramore GE, Austin-Seymour M, Koh WJ, Lindsley KL, Cho P and Griffin TW. Neutron radiotherapy for adenoid cystic carcinoma of minor salivary glands. International Journal of Radiation Oncology, Biology, Physics, Volume 36, Issue 1, 1 August 1996, Pages 87–93
- MacDougall RH, Orr JA, Kerr GR, and Duncan W. Fast neutron treatment for squamous cell carcinoma of the head and neck: final report of Edinburgh randomised trial. BMJ. 1990 December 1; 301(6763): 1241–1242.
- Asgarali S, Errington RD, Jones AS. The treatment of recurrence following fast neutron therapy for head and neck malignancy. Clin Otolaryngol Allied Sci. 1996 Jun;21(3):274-7.
- K.J. Stelzer, K.L. Lindsley, P.S. Cho, G.E. Laramore and T.W. Griffin. Fast Neutron Radiotherapy: The University of Washington Experience and Potential Use of Concomitant Boost with Boron Neutron Capture. Radiation Protection Dosimetry 70:471–475 (1997)
- The neutron-therapy saga: a cautionary tale – MedicalPhysicsWeb
- Brahme A, Eenmaa J, Lindback S, Montelius A, Wootton P. Neutron beam characteristics from 50 MeV protons on beryllium using a continuously variable multi-leaf collimator. Radiother Oncol. 1983 Aug;1(1):65–76.
- Farr JB. A compact multileaf collimator for conventional and intensity modulated fast neutron therapy Medical Physics April 2004 Volume 31, Issue 4, p. 951
- Farr JB, Maughan RL, Yudelev M, Blosser E, Brandon J, Horste T Compact multileaf collimator for conformal and intensity modulated fast neutron therapy: Electromechanical design and validation Medical Physics – September 2006 – Volume 33, Issue 9, pp. 3313–3320
- University of Washington (UW) Radiation Oncology Department
- Clinical Neutron Therapy System (CNTS) Archived July 20, 2011, at the Wayback Machine
- Farr, J. B., R. L. Maughan, et al. (2007). "Radiologic validation of a fast neutron multileaf collimator." Med Phys 34(9): 3475–3484.
- Santanam, L., T. He, et al. (2007). "Intensity modulated neutron radiotherapy for the treatment of adenocarcinoma of the prostate." Int J Radiat Oncol Biol Phys 68(5): 1546–1556.
- Cohen L and Lennox A. Midwest Institute for Neutron Therapy at Fermilab. International Journal of Radiation Oncology, Biology, Physics, Volume 34, Issue 1, 1 January 1996, Page 269
- Revival of a unique and proven cancer treatment, neutron therapy
- "About Us". NIU Institute for Neutron Therapy. Archived from the original on December 20, 2008. Retrieved April 24, 2010.
- "Neutrons Against Cancer" (PDF). NIU Institute for Neutron Therapy. Archived from the original (PDF) on November 4, 2009. Retrieved April 24, 2010.
- Neutron Therapy