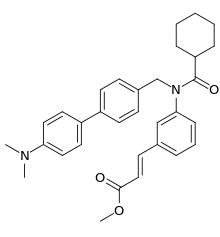
Fexaramine
Fexaramine is an investigational compound which acts as an agonist of the farnesoid X receptor (FXR), which is a bile acid-activated nuclear receptor that controls bile-acid synthesis, conjugation and transport, as well as lipid metabolism through actions in the liver and intestine.[1]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C32H36N2O3 |
| Molar mass | 496.651 g·mol−1 |
| 3D model (JSmol) | |
| |
The first publication about fexaramine in 2003 showed it has 100-fold greater affinity for FXR than natural compounds and described the genomic targets and binding site on FXR.[2]
When administered orally to mice, fexaramine produced selective actions through FXR receptors in the intestines.[3] Consistent with the effects of other FXR agonist drugs,[4] in a study in mice, oral fexaramine stimulated intestinal fibroblast growth factor 15 (FGF15) production and resulted in metabolic improvements. Intestinal tissue-specific actions of fexaramine were suggested to be a possible new approach for the treatment of obesity and metabolic syndrome.[3] However it cannot be determined from these preliminary results in mice whether FXR agonism with fexaramine will produce weight loss in humans. There are no clinical trials of fexaramine planned in humans and therapy with such FXR agonists for obesity is only a theoretical approach.[4]
References
- Claudel T, Sturm E, Kuipers F, Staels B (September 2004). "The farnesoid X receptor: a novel drug target?". Expert Opin Investig Drugs. 13 (9): 1135–48. doi:10.1517/13543784.13.9.1135. PMID 15330745.
- PDB: 1OSH; Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer JL, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JG, Noel JP, Nicolaou KC, Evans RM (April 2003). "A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR". Mol. Cell. 11 (4): 1079–92. doi:10.1016/S1097-2765(03)00104-7. PMC 6179153. PMID 12718892.
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM (January 2015). "Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance". Nat. Med. 21: 159–65. doi:10.1038/nm.3760. PMC 4320010. PMID 25559344.
- Ma H, Patti ME (August 2014). "Bile acids, obesity, and the metabolic syndrome". Best Pract Res Clin Gastroenterol. 28 (4): 573–83. doi:10.1016/j.bpg.2014.07.004. PMC 4159616. PMID 25194176.