Flavagline
Flavaglines are a family of natural products that are found in plants of the genus Aglaia (Meliaceae). These compounds are characterized by a cyclopenta[b]benzofuran skeleton. In 1982 King and colleagues discovered the first member of this family, rocaglamide, based on its antileukemic activity.[1] Since then, about 50 other flavaglines have been characterized. These molecules display strong insecticidal, antifungal, anti-inflammatory, neuroprotective, cardioprotective and anticancer activities.[2][3] In mouse models of cancer, flavaglines enhance the efficacy of chemotherapies[4][5] and also alleviate the cardiac adverse effect of these chemotherapies.[6]
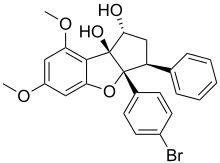
The challenge raised by their structural complexity has attracted the attention of some organic chemists. In 1990, Barry Trost presented an enantioselective synthesis of rocaglamide in 18 steps and confirmed its absolute configuration.[7]
See also
- FL3 (flavagline)
- Rocaglamide
- Silvestrol
- Aglafoline
References
- King, M. L.; Chiang, C. C.; Ling, H. C.; Fujita, E.; Ochiai, M.; McPhail, A. T. "X-Ray crystal structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia". J Chem Soc Chem Commun. 1982: 1150–1151.
- Ebada, S. S.; Lajkiewicz, N.; Porco, J. A. Jr.; Li-Weber, M.; Proksch, P. (2011). "Chemistry and biology of rocaglamides (= flavaglines) and related derivatives from aglaia species (meliaceae)". Fortschr Chem Org Naturst. Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. 94: 1–58. doi:10.1007/978-3-7091-0748-5_1. ISBN 978-3-7091-0747-8. PMC 4157394. PMID 21833837.
- Ribeiro, N.; Thuaud, F.; Nebigil, C.; Désaubry, L., Recent advances in the biology and chemistry of the flavaglines. Bioorg Med Chem 2012, in press.
- Bordeleau, M. E.; Robert, F.; Gerard, B.; Lindqvist, L.; Chen, S. M.; Wendel, H. G.; Brem, B.; Greger, H.; Lowe, S. W.; Porco, J. A. Jr.; Pelletier, J. (2008). "Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model" (PDF). J Clin Invest. 118 (7): 2651–60. doi:10.1172/JCI34753. PMC 2423864. PMID 18551192.
- Zhu, J. Y.; Giaisi, M.; Kohler, R.; Muller, W. W.; Muhleisen, A.; Proksch, P.; Krammer, P. H.; Li-Weber, M. (2009). "Rocaglamide sensitizes leukemic T cells to activation-induced cell death by differential regulation of CD95L and c-FLIP expression". Cell Death Differ. 16 (9): 1289–99. doi:10.1038/cdd.2009.42. PMID 19373244.
- Bernard, Y.; Ribeiro, N.; Thuaud, F.; Türkeri, G.; Dirr, R.; Boulberdaa, M.; Nebigil, C.; Désaubry, L. (2011). "Flavaglines Alleviate Doxorubicin Cardiotoxicity: Implication of Hsp27". PLoS ONE. 6 (10): e25302. Bibcode:2011PLoSO...625302B. doi:10.1371/journal.pone.0025302. PMC 3204970. PMID 22065986.
- Trost, B. M.; Greenspan, P. D.; Yang, B. V.; Saulnier, M. G. (1990). "An unusual oxidative cyclization. A synthesis and absolute stereochemical assignment of (−)-rocaglamide". J Am Chem Soc. 112 (24): 9022–4. doi:10.1021/ja00180a081.