Benzofuran
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Benzofuran[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 107704 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.439 | ||
| EC Number |
| ||
| 260881 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H6O | |||
| Molar mass | 118.135 g·mol−1 | ||
| Melting point | −18 °C (0 °F; 255 K) | ||
| Boiling point | 173 °C (343 °F; 446 K) | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Warning | |||
| H226, H351, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P273, P280, P281, P303+P361+P353, P308+P313, P370+P378, P403+P235, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
500 mg/kg (mice).[2] | ||
| Related compounds | |||
Related compounds |
Benzothiophene, Indole, Indene, 2-Cumaranone | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Production
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol.[2]
Laboratory methods
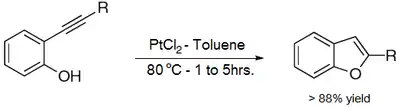
Benzofurans can be prepared by various methods in the laboratory. Notable examples include:
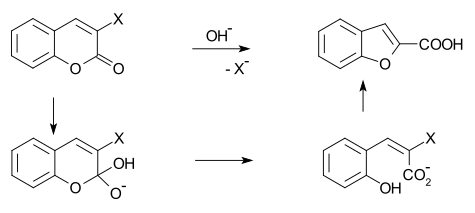
- O-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation.[3]

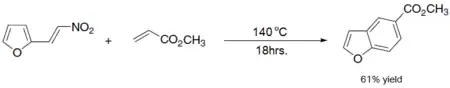
- Diels–Alder reaction of nitro vinyl furans with various dienophiles:[7]
Related compounds
- Substituted benzofurans
- Dibenzofuran, an analog with a second fused benzene ring.
- Furan, an analog without the fused benzene ring.
- Indole, an analog with a nitrogen instead of the oxygen atom.
- Benzothiophene, an analog with a sulfur instead of the oxygen atom.
- Isobenzofuran, the isomer with oxygen in the adjacent position.
- Aurone
- Thunberginol F
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 218. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Collin, G.; Höke, H. (2007). "Benzofurans". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.l03_l01. ISBN 978-3527306732.
- Burgstahler, A. W.; Worden, L. R. (1966). "Coumarone" (PDF). Organic Syntheses. 46: 28.; Collective Volume, vol. 5, p. 251
- Perkin, W. H. (1870). "XXIX. On some New Bromine Derivatives of Coumarin". Journal of the Chemical Society. 23: 368–371. doi:10.1039/JS8702300368.
- Perkin, W. H. (1871). "IV. On some New Derivatives of Coumarin". Journal of the Chemical Society. 24: 37–55. doi:10.1039/JS8712400037.
- Bowden, K.; Battah, S. (1998). "Reactions of Carbonyl Compounds in Basic Solutions. Part 32. The Perkin Rearrangement". Journal of the Chemical Society, Perkin Transactions 2. 1998 (7): 1603–1606. doi:10.1039/a801538d.
- Kusurkar, R. S.; Bhosale, D. K. (1990). "Novel Synthesis of Benzosubstituted Benzofurans Via Diels-Alder Reaction". Synthetic Communications. 20 (1): 101–109. doi:10.1080/00397919008054620.
- Fürstner, Alois & Davies, Paul (2005). "Heterocycles by PtCl2-Catalyzed Intramolecular Carboalkoxylation or Carboamination of Alkynes". Journal of the American Chemical Society. 127 (43): 15024–15025. doi:10.1021/ja055659p. hdl:11858/00-001M-0000-0025-AA5A-1. PMID 16248631.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.