Perkin rearrangement
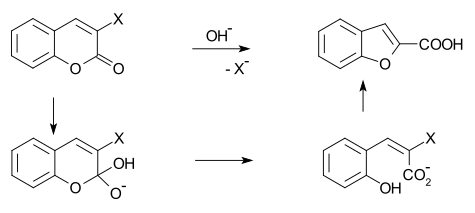
In organic chemistry, the Perkin rearrangement (coumarin–benzofuran ring contraction) is a rearrangement reaction in which a 2-halocoumarin in the presence of hydroxide undergoes a ring contraction to form a benzofuran. The name reaction recognizes William Henry Perkin, who first reported it in 1870. Several proposals have been made for the reaction mechanism, all of which involve initial opening of the lactone to give a carboxylate and phenolate.[1]
References
- Marriott, Karla-Sue C.; Bartee, Rena; Morrison, Andrew Z.; Stewart, Leonard; Wesby, Julian (2012). "Expedited Synthesis of Benzofuran-2-Carboxylic Acids via Microwave-Assisted Perkin Rearrangement Reaction". Tetrahedron Lett. 53 (26): 3319–3321. doi:10.1016/j.tetlet.2012.04.075. PMC 3377186. PMID 22736873.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.