Glycoside hydrolase family 30
In molecular biology, glycoside hydrolase family 30 is a family of glycoside hydrolases.
| O-Glycosyl hydrolase family 30 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
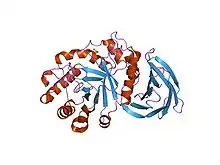 Structure of a xylanase from glycoside hydrolase family 5.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Glyco_hydro_30 | ||||||||
| Pfam | PF02055 | ||||||||
| Pfam clan | CL0058 | ||||||||
| InterPro | IPR001139 | ||||||||
| SCOP2 | 1nof / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 117 | ||||||||
| OPM protein | 1ogs | ||||||||
| |||||||||
Glycoside hydrolases EC 3.2.1. are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of >100 different families.[2][3][4] This classification is available on the CAZy web site,[5][6] and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes.[7][8]
Glycoside hydrolase family 30 CAZY GH_30 includes the mammalian glucosylceramidases. Human acid beta-glucosidase (D-glucosyl-N-acylsphingosine glucohydrolase), cleaves the glucosidic bonds of glucosylceramide and synthetic beta-glucosides.[9] Any one of over 50 different mutations in the gene of glucocerebrosidase have been found to affect activity of this hydrolase, producing variants of Gaucher disease, the most prevalent lysosomal storage disease.[9][10]
References
- Larson SB, Day J, Barba de la Rosa AP, Keen NT, McPherson A (July 2003). "First crystallographic structure of a xylanase from glycoside hydrolase family 5: implications for catalysis". Biochemistry. 42 (28): 8411–22. doi:10.1021/bi034144c. PMID 12859186.
- Henrissat B, Callebaut I, Fabrega S, Lehn P, Mornon JP, Davies G (July 1995). "Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases". Proceedings of the National Academy of Sciences of the United States of America. 92 (15): 7090–4. Bibcode:1995PNAS...92.7090H. doi:10.1073/pnas.92.15.7090. PMC 41477. PMID 7624375.
- Davies G, Henrissat B (September 1995). "Structures and mechanisms of glycosyl hydrolases". Structure. 3 (9): 853–9. doi:10.1016/S0969-2126(01)00220-9. PMID 8535779.
- Henrissat B, Bairoch A (June 1996). "Updating the sequence-based classification of glycosyl hydrolases". The Biochemical Journal. 316 (Pt 2): 695–6. doi:10.1042/bj3160695. PMC 1217404. PMID 8687420.
- "Home". CAZy.org. Retrieved 2018-03-06.
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (January 2014). "The carbohydrate-active enzymes database (CAZy) in 2013". Nucleic Acids Research. 42 (Database issue): D490-5. doi:10.1093/nar/gkt1178. PMC 3965031. PMID 24270786.
- "Glycoside Hydrolase Family 30". CAZypedia.org. Retrieved 2018-03-06.
- CAZypedia Consortium (December 2018). "Ten years of CAZypedia: a living encyclopedia of carbohydrate-active enzymes". Glycobiology. 28 (1): 3–8. doi:10.1093/glycob/cwx089. PMID 29040563.
- Dinur T, Osiecki KM, Legler G, Gatt S, Desnick RJ, Grabowski GA (March 1986). "Human acid beta-glucosidase: isolation and amino acid sequence of a peptide containing the catalytic site". Proceedings of the National Academy of Sciences of the United States of America. 83 (6): 1660–4. Bibcode:1986PNAS...83.1660D. doi:10.1073/pnas.83.6.1660. PMC 323143. PMID 3456607.
- Iwasawa K, Ida H, Eto Y (August 1997). "Differences in origin of the 1448C mutation in patients with Gaucher disease". Acta Paediatrica Japonica. 39 (4): 451–3. doi:10.1111/j.1442-200x.1997.tb03616.x. PMID 9316290. S2CID 26851772.