Hexafluorobutadiene
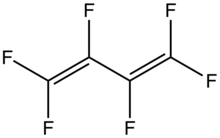
Hexafluorobutadiene is an organofluorine compound with the formula (CF2=CF)2. A colorless gas, it has attracted attention as an etchant in microelectronics. It is the perfluoroanalogue of butadiene.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexafluorobuta-1,3-diene | |
| Other names
1,1,2,3,4,4-Hexafluoro-1,3-butadiene, FC 2316 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.620 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4F6 | |
| Molar mass | 162.034 g·mol−1 |
| Appearance | colorless gas |
| Density | 1.44 g/cm3 (@15 °C) |
| Melting point | −132 °C (−206 °F; 141 K) |
| Boiling point | 6 °C (43 °F; 279 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H220, H280, H331 | |
| P210, P261, P271, P304+P340, P311, P321, P377, P381, P403, P403+P233, P405, P410+P403, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It can be prepared by coupling of C2 compounds such as from chlorotrifluoroethylene or bromotrifluoroethylene. Routes from C4 species have also been demonstrated. For example, an early synthesis involved Zn-induced dechlorinaion of 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluorobutane.
Hexafluorobutadiene dimerizes via a [2+2] process at 150 °C to give perfluorinated divinylcyclobutanes.[1]
See also
- Hexafluoro-2-butyne, an isomer of C4F6
- Hexafluorocyclobutene, an isomer of C4F6
- Hexachlorobutadiene
References
- Lemal, David M.; Chen, Xudong (2005). "Fluorinated Cyclobutanes and Their Derivatives". In Zvi Rappoport; Joel F. Liebman (eds.). The Chemistry of Cyclobutanes. PATAI'S Chemistry of Functional Groups. pp. 955–1029. doi:10.1002/0470864028.ch21. ISBN 0470864001.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.