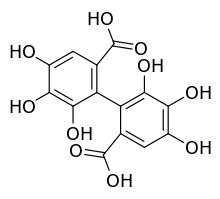
Hexahydroxydiphenic acid
Hexahydroxydiphenic acid is an organic compound with the formula [(HO)3C6HCO2H]2. It is the oxidatively coupled derivative of gallic acid[2] It is a white solid, although samples are typically brown owing to oxidation.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′,5,5′,6,6′-Hexahydroxy[1,1′-biphenyl]-2,2′-dicarboxylic acid | |
| Other names
HHDP 3,4,5,3′,4′,5′-Hexahydroxydiphenate 3,4,5,3′,4′,5′-Hexahydroxydiphenic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C14H10O10 | |
| Molar mass | 338.224 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
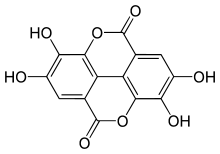
Hexahydroxydiphenic acid is a component of some ellagitannins,[3] such as casuarictin. Luteic acid is the monolactone and ellagic acid is the dilactone of hexahydroxydiphenic acid.
See also
References
- "MetaCyc hexahydroxydiphenic acid". biocyc.org.
- Haslam, E.; Cai, Y. (1994). "Plant polyphenols (vegetable tannins): Gallic acid metabolism". Natural Product Reports. 11 (1): 41–66. doi:10.1039/NP9941100041. PMID 15206456.
- Feldman, Ken S.; Iyer, Malliga R.; Liu, Yanze (2003). "Ellagitannin Chemistry. Studies on the Stability and Reactivity of 2,4-HHDP-Containing Glucopyranose Systems". The Journal of Organic Chemistry. 68 (19): 7433–7438. doi:10.1021/jo034495x. PMID 12968897.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.