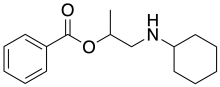
Hexylcaine
Hexylcaine hydrochloride, also called cyclaine (Merck) or osmocaine, is a short-acting local anesthetic. It acts by inhibiting sodium channel conduction. Overdose can lead to headache, tinnitus, numbness and tingling around the mouth and tongue, convulsions, inability to breathe, and decreased heart function.[1]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | <10 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.365 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
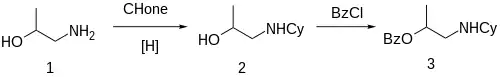
Synthesis
The reductive amination between 1-Amino-2-propanol [78-96-6] (1) and cyclohexanone gives 1-Cyclohexylamino-2-propanol [103-00-4] (2). Treatment with benzoyl chloride gives the ester, completing the synthesis of Hexylcaine (3).
References
- Spellberg MA (January 1959). "Hexylcaine (cyclaine) as topical anesthetic in gastroscopy and esophagoscopy". Gastroenterology. 36 (1): 120–1. doi:10.1016/S0016-5085(59)80102-5. PMID 13620024.
- Cope, Arthur C.; Hancock, Evelyn M. (1944). "1-Alkylamino-2-propanols and their p-Nitro- and p-Aminobenzoates". Journal of the American Chemical Society 66 (9): 1453–1456. doi:10.1021/ja01237a010.
- "Local Anesthetics". New England Journal of Medicine. 263 (19): 963–965.1960. doi:10.1056/NEJM196011102631912.
- Cope Arthur C, U.S. Patent 2,486,374 (1949 to Sharp & Dohme Inc).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.