HslVU
The heat shock proteins HslV and HslU (HslVU complex; also known as ClpQ and ClpY respectively, or ClpQY) are expressed in many bacteria such as E. coli in response to cell stress.[1] The hslV protein is a protease and the hslU protein is an ATPase; the two form a symmetric assembly of four stacked rings, consisting of an hslV dodecamer bound to an hslU hexamer, with a central pore in which the protease and ATPase active sites reside. The hslV protein degrades unneeded or damaged proteins only when in complex with the hslU protein in the ATP-bound state. HslV is thought to resemble the hypothetical ancestor of the proteasome, a large protein complex specialized for regulated degradation of unneeded proteins in eukaryotes, many archaea, and a few bacteria. HslV bears high similarity to core subunits of proteasomes.[2]
| HslU—HslV peptidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
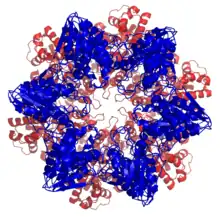 Top view of the hslV/hslU complex isolated from E. coli (PDB ID 1G4A). | |||||||||
| Identifiers | |||||||||
| EC no. | 3.4.25.2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
| Heat shock protein HslU | |
|---|---|
| Identifiers | |
| Symbol | HslU |
| InterPro | IPR004491 |
| ATP-dependent protease, HslV subunit | |
|---|---|
| Identifiers | |
| Symbol | HslV |
| InterPro | IPR022281 |
Genetics
Both proteins are encoded on the same operon within the bacterial genome. Unlike many eukaryotic proteasomes, which have several different peptide substrate specificities, hslV has a specificity similar to that of chymotrypsin; hence it is inhibited by proteasome inhibitors that specifically target the chymotrypsin site in eukaryotic proteasomes.[3] Although the HslVU complex is stable on its own, some evidence suggests that the complex is formed in vivo in a substrate-induced manner due to a conformational change in the hslU-substrate complex that promotes hslV binding.[4]
HslV and hslU genes have also been identified in some eukaryotes, although these also require the constitutively expressed proteasome for survival. These eukaryotic HslVU complexes assemble to apparently functional units, suggesting that these eukaryotes have both functional proteasomes and functional hslVU systems.[5]
Regulation
The promoter region of the operon encoding HslU and HslV contains a stem-loop structure which is necessary for gene expression. This structure contributes to mRNA stability.[6]
Motifs in peptide unfolding
A four-amino acid sequence motif - GYVG, glycine-tyrosine-valine-glycine - conserved in hslU ATPases and located on the inner surface of the assembled pore dramatically accelerates the degradation of some proteins, and is required for the degradation of others. However, these motifs are not necessary for the degradation of short peptides and play no direct role in hydrolysis, suggesting that their major role is in unfolding the native state structure of the substrate and transferring the resulting disordered polypeptide chain to the hslV subunits for degradation. These motifs also influence the assembly of the complex.[7] Translocation is also facilitated by the C-terminal tails of the HslU subunits, which form a gate closing off the proteolytic active sites in the central pore until a substrate has been bound and unfolded.[8]
Mechanism
The basic mechanism by which the hslVU complex undertakes proteolytic substrate degradation is essentially the same as that observed in the eukaryotic proteasome, catalyzed by Nactive-site threonine residues. Both are members of the T1 family.[9] It is inhibited by enzyme inhibitors that covalently bind the threonine.[10] Like the proteasome, hslU must bind ATP in a magnesium-dependent manner before substrate binding and unfolding can occur.[11]
References
- Ramachandran R, Hartmann C, Song HK, Huber R, Bochtler M. (2002). Functional interactions of HslV (ClpQ) with the ATPase HslU (ClpY). Proc Natl Acad Sci USA 99(11):7396-401.
- Gille C, Goedel A, Schloetelburg C, Preißner R, Kloetzell PM, Gobel UB, Frommell C. (2003). A Comprehensive View on Proteasomal Sequences: Implications for the Evolution of the Proteasome. J Mol Biol 326: 1437–1448.
- Rohrwild M, Coux O, Huang HC, R P Moerschell RP, Yoo SJ, Seol JH, Chung CH, Goldberg AL. (1996). HslV-HslU: A novel ATP-dependent protease complex in Escherichia coli related to the eukaryotic proteasome. Proc Natl Acad Sci USA 93(12): 5808–5813
- Azim MK, Goehring W, Song HK, Ramachandran R, Bochtler M, Goettig P. (2005). Characterization of the HslU chaperone affinity for HslV protease. Protein Sci 14(5):1357-62.
- Ruiz-Gonzalez MX, Marin I. (2006). Proteasome-related HslU and HslV genes typical of eubacteria are widespread in eukaryotes. J Mol Evol 63(4):504-12.
- Lien, HY; Yu, CH; Liou, CM; Wu, WF (2009). "Regulation of clpQ⁺Y⁺ (hslV⁺U⁺) gene expression in Escherichia coli". The Open Microbiology Journal. 3: 29–39. doi:10.2174/1874285800903010029. PMC 2681174. PMID 19440251.
- Park E, Rho YM, Koh OJ, Ahn SW, Seong IS, Song JJ, Bang O, Seol JH, Wang J, Eom SH, Chung CH. (2005). Role of the GYVG pore motif of HslU ATPase in protein unfolding and translocation for degradation by HslV peptidase. J Biol Chem 280(24):22892-8.
- Seong IS, Kang MS, Choi MK, Lee JW, Koh OJ, Wang J, Eom SH, Chung CH. (2002). The C-terminal tails of HslU ATPase act as a molecular switch for activation of HslV peptidase. J Biol Chem 277(29):25976-82.
- Bogyo M, McMaster JS, Gaczynska M, Tortorella D, Goldberg AL, Ploegh H. (1997). Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc Natl Acad Sci USA 94(13):6629-34.
- Sousa MC, Kessler BM, Overkleeft HS, McKay DB. (2002). Crystal structure of HslUV complexed with a vinyl sulfone inhibitor: corroboration of a proposed mechanism of allosteric activation of HslV by HslU. J Mol Biol 318(3):779-85.
- Burton RE, Baker TA, Sauer RT. (2005). Nucleotide-dependent substrate recognition by the AAA+ HslUV protease. Nat Struct Mol Biol 12(3):245-51.
External links
- HslU---HslV+peptidase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
Further reading
- Wang J, Rho SH, Park HH, Eom SH (July 2005). "Correction of X-ray intensities from an HslV-HslU co-crystal containing lattice-translocation defects". Acta Crystallographica Section D. 61 (Pt 7): 932–41. doi:10.1107/s0907444905009546. PMID 15983416.
- Nishii W, Takahashi K (October 2003). "Determination of the cleavage sites in SulA, a cell division inhibitor, by the ATP-dependent HslVU protease from Escherichia coli". FEBS Letters. 553 (3): 351–4. doi:10.1016/s0014-5793(03)01044-5. PMID 14572649.
- Ramachandran R, Hartmann C, Song HK, Huber R, Bochtler M (May 2002). "Functional interactions of HslV (ClpQ) with the ATPase HslU (ClpY)". Proceedings of the National Academy of Sciences of the United States of America. 99 (11): 7396–401. doi:10.1073/pnas.102188799. PMC 124242. PMID 12032294.
- Yoo SJ, Seol JH, Shin DH, Rohrwild M, Kang MS, Tanaka K, Goldberg AL, Chung CH (June 1996). "Purification and characterization of the heat shock proteins HslV and HslU that form a new ATP-dependent protease in Escherichia coli". The Journal of Biological Chemistry. 271 (24): 14035–40. doi:10.1074/jbc.271.24.14035. PMID 8662828.
- Yoo SJ, Seol JH, Seong IS, Kang MS, Chung CH (September 1997). "ATP binding, but not its hydrolysis, is required for assembly and proteolytic activity of the HslVU protease in Escherichia coli". Biochemical and Biophysical Research Communications. 238 (2): 581–5. doi:10.1006/bbrc.1997.7341. PMID 9299555.
- Kanemori M, Nishihara K, Yanagi H, Yura T (December 1997). "Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of sigma32 and abnormal proteins in Escherichia coli". Journal of Bacteriology. 179 (23): 7219–25. doi:10.1128/jb.179.23.7219-7225.1997. PMC 179669. PMID 9393683.
- Burton RE, Baker TA, Sauer RT (March 2005). "Nucleotide-dependent substrate recognition by the AAA+ HslUV protease". Nature Structural & Molecular Biology. 12 (3): 245–51. doi:10.1038/nsmb898. PMID 15696175. S2CID 28864309.</ref>