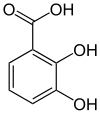
2,3-Dihydroxybenzoic acid
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus[2] and in the aquatic fern Salvinia molesta.[3] It is also abundant in the fruits of Flacourtia inermis. It is a dihydroxybenzoic acid, a type of organic compound.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydroxybenzoic acid | |
| Other names
Hypogallic acid; 2-Pyrocatechuic acid; o-Pyrocatechuic acid | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | 2,3-DHBA; 2,3-DHB |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.582 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H6O4 | |
| Molar mass | 154.121 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.542 g/cm3 (20 °C)[1] |
| Melting point | 205 °C (401 °F; 478 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The colorless solid occurs naturally, being formed via the shikimate pathway. It is incorporated into various siderophores, which are molecules that strongly complex iron ions for absorption into bacteria. 2,3-DHB consists of a catechol group, which upon deprotonation binds iron centers very strongly, and the carboxylic acid group by which the ring attaches to various scaffolds through amide bonds. A famous high affinity siderophore is enterochelin, which contains three dihydroxybenzoyl substituents linked to the depsipeptide of serine.[4][5]
It is a potentially useful iron-chelating drug[6] and has antimicrobial properties.[7][8][9]
2,3-Dihydroxybenzoic acid is also a product of human aspirin metabolism.[10]
References
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.190. ISBN 9781498754293.
- Sousa M, Ousingsawat J, Seitz R, et al. (January 2007). "An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: A potential treatment for cystic fibrosis". Mol. Pharmacol. 71 (1): 366–76. doi:10.1124/mol.106.025262. PMID 17065237. S2CID 5793585.
- Choudhary, M. I.; Naheed, N.; Abbaskhan, A.; Musharraf, S. G.; Siddiqui, H.; Atta-Ur-Rahman (2008). "Phenolic and other constituents of fresh water fern Salvinia molesta". Phytochemistry. 69 (4): 1018–1023. doi:10.1016/j.phytochem.2007.10.028. PMID 18177906.
- O'Brien, I. G.; Cox, G. B.; Gibson, F. (1970). "Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli". Biochimica et Biophysica Acta (BBA) - General Subjects. 201 (3): 453–60. doi:10.1016/0304-4165(70)90165-0. PMID 4908639.
- Young, I. G.; Gibson, F. (1969). "Regulation of the enzymes involved in the biosynthesis of 2,3-dihydroxybenzoic acid in Aerobacter aerogenes and Escherichia coli". Biochimica et Biophysica Acta (BBA) - General Subjects. 177 (3): 401–11. doi:10.1016/0304-4165(69)90302-X. PMID 4306838.
- Graziano, J. H.; Grady, R. W.; Cerami, A. (1974). "The identification of 2,3-dihroxybenzoic acid as a potentially useful iron-chelating drug". Journal of Pharmacology and Experimental Therapeutics. 190 (3): 570–575. PMID 4416298.
- George, Shibumon; PJ, Benny; Kuriakose, Sunny; George, Cincy (2011). "Antibiotic activity of 2, 3-dihydroxybenzoic acid isolated from Flacourtia inermis fruit against multidrug resistant bacteria" (PDF). Asian Journal of Pharmaceutical and Clinical Research. 4 (1).
- PJ, Benny; Shibumon, George; Sunny, Kuriakose; Cincy, George (2010). "2, 3-Dihydroxybenzoic Acid: An Effective Antifungal Agent Isolated from Flacourtia inermis Fruit". International Journal of Pharmaceutical and Clinical Research. 2 (3): 101–105.
- George, Shibumon; PJ, Benny; Kuriakose, Sunny; George, Cincy; Sarala, Gopalakrishnan (2011). "Antiprotozoal activity of 2, 3-dihydroxybenzoic acid isolated from the fruit extracts of Flacourtia inermis Roxb". Medicinal Plants - International Journal of Phytomedicines and Related Industries. 3 (3): 237–241. doi:10.5958/j.0975-4261.3.3.038.
- Grootveld, M.; Halliwell, B. (1988). "2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism". Biochemical Pharmacology. 37 (2): 271–280. doi:10.1016/0006-2952(88)90729-0. PMID 3342084.