Ice XII
Ice XII is a metastable, dense, crystalline phase of solid water, a type of ice. Ice XII was first reported in 1996 by C. Lobban, J.L. Finney and W.F. Kuhs and, after initial caution, was properly identified in 1998.
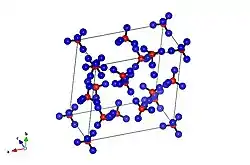
It was first obtained by cooling liquid water to 260 K (−13 °C; 8 °F) at a pressure of 0.55 gigapascals (5,400 atm). Ice XII was discovered existing within the phase stability region of ice V. Later research showed that ice XII could be created outside that range. Pure ice XII can be created from ice Ih at 77 K (−196.2 °C; −321.1 °F) by rapid compression (0.81-1.00 GPa/min) or by warming high density amorphous ice at pressures between 0.8 to 1.6 gigapascals (7,900 to 15,800 atm).
While it is similar in density (1.29 g/cm3 at 127 K (−146 °C; −231 °F)) to ice IV (also found in the ice V space) it exists as a tetragonal crystal. Topologically it is a mix of seven- and eight-membered rings, a 4-connected net (4-coordinate sphere packing)—the densest possible arrangement without hydrogen bond interpenetration.
Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XVI, have been created in the laboratory at different temperatures and pressures.
Ice XIV
When hydrochloric-acid-doped ice XII is cooled down to about 110 K, it undergoes a phase transition into a partially hydrogen-ordered phase, namely ice XIV.[1] The transition entropy from ice XIV to ice XII is estimated to be 60% of Pauling entropy based on DSC measurements.[2] The formation of ice XIV from ice XII is more favoured at high pressure.[3]
See also
- Ice for other crystalline form of ice
References
- Salzmann CG, Radaelli PG, Hallbrucker A, Mayer E, Finney JL (2006). "The preparation and structures of hydrogen ordered phases of ice". Science. 311 (5768): 1758–61. Bibcode:2006Sci...311.1758S. doi:10.1126/science.1123896. PMID 16556840. S2CID 44522271.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Köster KW, Fuentes-Landete V, Raidt A, Seidl M, Gainaru C, Loerting T; et al. (2018). "Author Correction: Dynamics enhanced by HCl doping triggers 60% Pauling entropy release at the ice XII-XIV transition". Nat Commun. 9: 16189. Bibcode:2018NatCo...916189K. doi:10.1038/ncomms16189. PMC 6026910. PMID 29923547.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fuentes-Landete V; Köster KW; Böhmer R; Loerting T (2018). "Thermodynamic and kinetic isotope effects on the order-disorder transition of ice XIV to ice XII". Phys Chem Chem Phys. 20 (33): 21607–21616. Bibcode:2018PCCP...2021607F. doi:10.1039/c8cp03786h. PMID 30101255. S2CID 51969440.
- C. Lobban, J.L. Finney and W.F. Kuhs, The structure of a new phase of ice, Nature 391, 268–270, 1998
- Chaplin, Martin (2007-07-01). "Ice-twelve and ice-fourteen structures". Water Structure and Science. Retrieved 2008-01-02.