Enasidenib
Enasidenib (INN; trade name Idhifa) is a medication used to treat relapsed or refractory acute myeloid leukemia in people with specific mutations of the isocitrate dehydrogenase 2 (IDH2) gene, determined by an FDA-approved IDH2 companion diagnostic test.[2] It is an inhibitor of IDH2. It was developed by Agios Pharmaceuticals and is licensed to Celgene for further development.
 | |
| Clinical data | |
|---|---|
| Trade names | Idhifa |
| Other names | AG-221 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617040 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
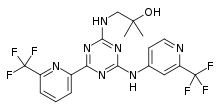
| Formula | C19H17F6N7O |
| Molar mass | 473.383 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[3]
Medical use
Enasidenib is used to treat relapsed or refractory acute myeloid leukemia in people with specific mutations of the IDH2 gene, determined by an FDA-approved IDH2 companion diagnostic test.[2]
Adverse effects
The main serious adverse effect of enasidenib is differentiation syndrome.[4]
Pharmacology
Isocitrate dehydrogenase is a critical enzyme in the citric acid cycle. Mutated forms of IDH produce high levels of the (R)-enantiomer of 2-hydroxyglutarate (R-2-HG) and can contribute to the growth of tumors. IDH1 catalyzes this reaction in the cytoplasm, while IDH2 catalyzes this reaction in mitochondria. Mutations of IDH2 are more common than IDH1 mutations, 8 to 19% compared to 7 to 14% respectively,[2] in those affected with AML. Enasidenib disrupts this cycle by decreasing total (R)-2-HG levels in the mitochondria.
History
The U.S. Food and Drug Administration (FDA) granted the application for enasidenib fast track designation and orphan drug designation for acute myeloid leukemia in 2014.[4]
Enasidenib was approved by the FDA in August 2017, for relapsed or refractory acute myeloid leukemia (AML) in people with specific mutations of the IDH2 gene, determined by an FDA-approved IDH2 companion diagnostic test.[2][5][6]
References
- "Summary Basis of Decision (SBD) for Idhifa". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- Kim ES (October 2017). "Enasidenib: First Global Approval". Drugs. 77 (15): 1705–1711. doi:10.1007/s40265-017-0813-2. PMID 28879540. S2CID 7685848.
- New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
- Brunton LL, Hilal-Dandan R, Knollmann BC (5 December 2017). Goodman & Gilman's the pharmacological basis of therapeutics (13th ed.). New York: McGraw Hill Education. ISBN 9781259584732. OCLC 993810322.
- "FDA Approves New Treatment for Leukemia". Genetic Engineering & Biotechnology News (GEN). August 2, 2017.
- "Press release: FDA granted regular approval to enasidenib for the treatment of relapsed or refractory AML". FDA. August 1, 2017.
External links
- "Enasidenib". Drug Information Portal. U.S. National Library of Medicine.
- "Enasidenib mesylate". NCI Dictionary of Cancer Terms. National Cancer Institute.
- "Enasidenib mesylate". National Cancer Institute. 8 August 2017.