Inositol polyphosphate kinase
Inositol polyphosphate kinase (IPK) is a family of enzymes[1] that have a similar 3-dimensional structure. All members of the family catalyze the transfer of phosphate groups from ATP to various inositol phosphates. Members of the family include inositol-polyphosphate multikinases, inositol-hexakisphosphate kinases, inositol-trisphosphate 3-kinases, and inositol-pentakisphosphate 2-kinase, which is more distantly related to the others[2][3]
| Inositol polyphosphate kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
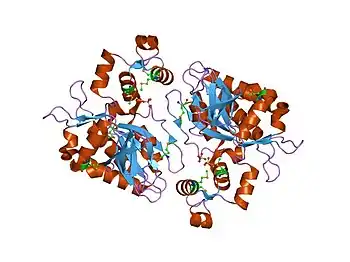 Structure of the Inositol 1,4,5-trisphosphate 3-kinase A protein. | |||||||||
| Identifiers | |||||||||
| Symbol | IPK | ||||||||
| Pfam | PF03770 | ||||||||
| InterPro | IPR005522 | ||||||||
| SCOP2 | 1tzd / SCOPe / SUPFAM | ||||||||
| |||||||||
The discovery of the IPK family occurred in 1999, when a combination of biochemistry, sequence analysis, and genetics led to the classification of a family of inositol polyphosphate kinases. [4][5] In 2005, the first crystal structures of an IPK family protein were published for ITPKA.[6][7]
Subsequently, structures of the inositol polyphosphate multikinase and various IP6 kinases have expanded our structural understanding for how each enzyme catalyzes its specific reaction(s).
References
- "Kinase Family IPK - WikiKinome". kinase.com.
- "InterPro".
- González B, Baños-Sanz JI, Villate M, Brearley CA, Sanz-Aparicio J (2010). "Inositol 1,3,4,5,6-pentakisphosphate 2-kinase is a distant IPK member with a singular inositide binding site for axial 2-OH recognition". Proc Natl Acad Sci U S A. 107 (21): 9608–13. Bibcode:2010PNAS..107.9608G. doi:10.1073/pnas.0912979107. PMC 2906834. PMID 20453199.
- Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH (1999). "Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases". Curr Biol. 9 (22): 1323–6. doi:10.1016/s0960-9822(00)80055-x. PMID 10574768.
- Odom AR, Stahlberg A, Wente SR, York JD (2000). "A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control". Science. 287 (5460): 2026–9. Bibcode:2000Sci...287.2026O. doi:10.1126/science.287.5460.2026. PMID 10720331.
- González B, Schell MJ, Letcher AJ, Veprintsev DB, Irvine RF, Williams RL (2004). "Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase". Mol Cell. 15 (5): 689–701. doi:10.1016/j.molcel.2004.08.004. PMID 15350214.
- Miller GJ, Hurley JH (2004). "Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase". Mol Cell. 15 (5): 703–11. doi:10.1016/j.molcel.2004.08.005. PMID 15350215.