Ertapenem
Ertapenem, sold under the brand name Invanz, is a carbapenem antibiotic medication used for the treatment of infections of the abdomen, the lungs, the upper part of the female reproductive system, and the diabetic foot.[7][8]
 | |
| Clinical data | |
|---|---|
| Trade names | Invanz |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614001 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% (intramuscular) |
| Protein binding | Inversely proportional to concentration; 85 to 95% |
| Metabolism | Hydrolysis of beta-lactam ring, CYP not involved |
| Elimination half-life | 4 hours |
| Excretion | Kidney (80%) and fecal (10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
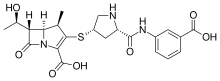
| Formula | C22H25N3O7S |
| Molar mass | 475.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The most common side effects include diarrhoea, nausea (feeling sick), headache, and problems around the area where the medicine is infused. It can significantly reduce the concentrations of valproic acid, an anti-seizure medication, in the blood to the point where it loses its effectiveness.[6]
Ertapenem was approved for medical use in the United States in November 2001,[5][9] and in the European Union in April 2002.[6] It is marketed by Merck.[5][6]
Medical uses
Ertapenem is indicated for the treatment of intra-abdominal infections, community-acquired pneumonia, pelvic infections, and diabetic foot infections, with bacteria that are susceptible to this drug, or expected to be so. It can also be used to prevent infections after colorectal surgery. In the United States it is also indicated for the treatment of complicated urinary tract infections including pyelonephritis.[5][7][10] It is a potential effective alternative treatment for ceftriaxone-resistant gonorrhoea.[11][12]
It is given as an intravenous infusion or intramuscular injection. The drug is not approved for children under three months of age.[5][7][10]
Contraindications
The drug is contraindicated in people with known hypersensitivity to ertapenem or other carbapenem type antibiotics, or with severe hypersensitivity reactions (such as anaphylaxis or severe skin reactions) to other beta-lactam antibiotics in the past.[5][7][10]
Side effects
Common side effects are diarrhoea (in 5% of people receiving ertapenem), nausea (in 3%) and vomiting, reactions at the injection site (5%, including pain and inflammation of the vein), and headache. Uncommon but possibly serious side effects include candida infections, seizures, skin reactions such as rashes (including nappy rash in children), and anaphylaxis.[10][13] Hypersensitivity cross-reactions with penicillins are rare.[14]
Ertapenem also can have an effect on some blood tests such as liver enzymes and platelet count.[7][10]
Overdose
Overdosing is unlikely. In adults receiving the threefold therapeutic dose over eight days, no significant toxicity was observed.[10]
Interactions
Ertapenem can reduce the concentrations of valproic acid, an epilepsy medication, by 70% and perhaps up to 95% within 24 hours; this can result in inadequate control of seizures.[13][15] The effect is described for other carbapenem antibiotics as well, but seems to be most pronounced for ertapenem and meropenem.[15] This is likely caused by several mechanisms: carbapenems inhibit transport of valproic acid from the gut into the body; they may increase metabolization of valproic acid to its glucuronide; they may reduce enterohepatic circulation and recycling of valproic acid glucuronide by acting against gut bacteria; and they may block transporter proteins that pump valproic acid out of red blood cells into the blood plasma.[16][17] The effect is also seen in reverse: in cases where ertapenem has been withdrawn blood concentrations of valproate have been reported to rise.[18][19]
Drug interactions via the cytochrome P450 enzyme system or the P-glycoprotein transporter are considered unlikely, as these proteins are not involved in the metabolism of ertapenem.[10]
Pharmacology
Mechanism of action
Like all beta-lactam antibiotics, ertapenem is bactericidal.[14] It inhibits cross-linking of the peptidoglycan layer of bacterial cell walls by blocking a type of enzymes called penicillin-binding proteins (PBPs). When a bacterial cell tries to synthesize new cell wall in order to grow and divide, the attempt fails, rendering the cell vulnerable to osmotic disruption. Additionally, the surplus of peptidoglycan precursors triggers autolytic enzymes of the bacterium, which disintegrate the existing wall.[20]
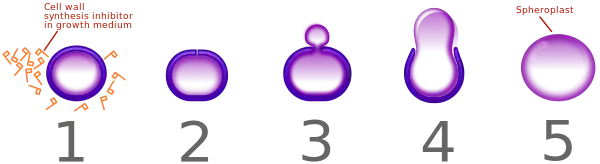
Susceptible bacteria
Bacteria that are normally susceptible to ertapenem treatment (at least in Europe) include:[10]
- Gram-positive aerobes
- Methicillin-susceptible Staphylococcus species (including Staphylococcus aureus)
- Streptococcus agalactiae
- Streptococcus pneumoniae (not established for penicillin-resistant strains)
- Gram-negative aerobes
- Anaerobes:
- Clostridium species (excluding C. difficile)
- Eubacterium species
- Fusobacterium species
- Peptostreptococcus species
- Porphyromonas asaccharolytica
- Prevotella species
- The US Food and Drug Administration (FDA) label specifies activity against additional anaerobes:[5]
- Bacteroides distasonis
- Bacteroides fragilis
- Bacteroides ovatus
- Bacteroides thetaiotaomicron
- Bacteroides uniformis
Resistance
Bacteria that show no clinically relevant response to ertapenem include methicillin-resistant Staphylococcus species (including MRSA) as well as Acinetobacter, Aeromonas, Enterococcus, and Pseudomonas.[10][14]
Microorganisms can become resistant to ertapenem by producing carbapenemases, enzymes that inactivate the drug by opening the beta-lactam ring. Other mechanisms of resistance against carbapenems are development of efflux pumps that transport the antibiotics out of the bacterial cells, mutations of PBPs, and mutations of Gram-negative bacteria's porins which are necessary for carbapenems to enter the bacteria.[8]
Pharmacokinetics
The route of administration has only a slight effect on the drug's concentrations in the bloodstream: when given as an intramuscular injection, its bioavailability is 90% (as compared to the 100% availability when given directly into a vein), and its highest concentrations in the blood plasma are reached after about 2.3 hours. In the blood, 85–95% of ertapenem are bound to plasma proteins, mostly albumin. Plasma protein binding is higher for lower concentrations, and vice versa. The drug is only partially metabolized, with 94% circulating in form of the parent substance and 6% as metabolites. The main metabolite is the inactive hydrolysis product with the ring opened.[7]
Ertapenem is mainly eliminated via the kidneys and urine (80%) and to a minor extent via the faeces (10%). Of the 80% found in the urine, 38% is excreted as the parent drug and 37% as the ring-opened metabolite. The biological half-life is about 3.5 hours in women, 4.2 hours in men and 2.5 hours in children up to 12 years of age.[7][13]
Comparison with other antibiotics
Like all carbapenem antibiotics, ertapenem has a broader spectrum of activity than other beta-lactams like penicillins and cephalosporins. Similar to doripenem, meropenem and biapenem, ertapenem has slightly better activity against many Gram-negative bacteria than other carbapenems such as imipenem. In contrast to imipenem, doripenem and meropenem, it is not active against Enterococcus, Pseudomonas and Acinetobacter species.[8][14]
For diabetic foot infections, ertapenem as a single treatment or in combination with vancomycin has been found to be more effective and have fewer side effects than tigecycline, but in severe cases it is less effective than piperacillin/tazobactam.[23][24]
Regarding pharmacokinetics, imipenem, doripenem and meropenem have lower plasma protein bindings (up to 25%) and shorter half-lives (about one hour) than ertapenem.[14]
History
Ertapenem is marketed by Merck. It was approved for use by the US Food and Drug Administration in November 2001,[9] and by the European Medicines Agency in April 2002.[6][25]
References
- "Ertapenem (Invanz) Use During Pregnancy". Drugs.com. 24 January 2020. Retrieved 29 July 2020.
- "INVANZ ertapenem (as sodium) 1g powder for injection vial (81449)". Therapeutic Goods Administration (TGA). 12 August 2022. Retrieved 21 April 2023.
- "TGA eBS - Product and Consumer Medicine Information Licence".
- "Invanz 1g powder for concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). Retrieved 29 July 2020.
- "Invanz- ertapenem sodium injection, powder, lyophilized, for solution". DailyMed. 13 February 2020. Retrieved 29 July 2020.
- "Invanz EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 29 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Ertapenem Sodium Monograph for Professionals". Drugs.com. The American Society of Health-System Pharmacists. 29 June 2020. Retrieved 29 July 2020.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA (November 2011). "Carbapenems: past, present, and future". Antimicrobial Agents and Chemotherapy. 55 (11): 4943–4960. doi:10.1128/AAC.00296-11. PMC 3195018. PMID 21859938.
- "Drug Approval Package: Invanz I.V. or I.M. (Ertapenem Sodium) NDA #21-337". U.S. Food and Drug Administration (FDA). 20 November 2001. Retrieved 29 July 2020.
- "Invanz: EPAR – Product Information" (PDF). European Medicines Agency. 19 November 2019.
- de Vries HJ, de Laat M, Jongen VW, Heijman T, Wind CM, Boyd A, et al. (May 2022). "Efficacy of ertapenem, gentamicin, fosfomycin, and ceftriaxone for the treatment of anogenital gonorrhoea (NABOGO): a randomised, non-inferiority trial". The Lancet. Infectious Diseases. 22 (5): 706–717. doi:10.1016/S1473-3099(21)00625-3. PMID 35065063. S2CID 246129045.
- "Antibiotic Ertapenem is alternative drug in treatment of gonorrhea". Amsterdam UMC. 20 January 2022. Retrieved 8 July 2022.
- "Ertapenem". mediQ. Retrieved 29 July 2020.
- Mutschler E (2013). Arzneimittelwirkungen (in German) (10th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 740, 753. ISBN 978-3-8047-2898-1.
- Wu CC, Pai TY, Hsiao FY, Shen LJ, Wu FL (October 2016). "The Effect of Different Carbapenem Antibiotics (Ertapenem, Imipenem/Cilastatin, and Meropenem) on Serum Valproic Acid Concentrations". Therapeutic Drug Monitoring. 38 (5): 587–592. doi:10.1097/FTD.0000000000000316. PMID 27322166. S2CID 25445129.
- Mancl EE, Gidal BE (December 2009). "The effect of carbapenem antibiotics on plasma concentrations of valproic acid". The Annals of Pharmacotherapy. 43 (12): 2082–2087. doi:10.1345/aph.1M296. PMID 19934386. S2CID 207263641.
- Arzneimittel-Interaktionen (in German). Österreichischer Apothekerverlag. 2019. p. 760. ISBN 978-3-85200-254-5.
- Zaccara G (31 December 2012). "Antiepileptic drugs". In Aronson JK (ed.). Side Effects of Drugs Annual 34: A worldwide yearly survey of new data in adverse drug reactions. Newnes. p. 121. ISBN 978-0-444-59503-4.
- Liao FF, Huang YB, Chen CY (August 2010). "Decrease in serum valproic acid levels during treatment with ertapenem". American Journal of Health-System Pharmacy. 67 (15): 1260–1264. doi:10.2146/ajhp090069. PMID 20651316.
- "Beta lactam antibiotics". StatPearls. 2020. PMID 31424895.
- Cushnie TP, O'Driscoll NH, Lamb AJ (December 2016). "Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action". Cellular and Molecular Life Sciences. 73 (23): 4471–4492. doi:10.1007/s00018-016-2302-2. hdl:10059/2129. PMID 27392605. S2CID 2065821.
- CID 45105276 from PubChem. Accessed 30 July 2020.
- Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA (September 2015). "Systemic antibiotics for treating diabetic foot infections". The Cochrane Database of Systematic Reviews. 2015 (9): CD009061. doi:10.1002/14651858.CD009061.pub2. PMC 8504988. PMID 26337865.
- Tchero H, Kangambega P, Noubou L, Becsangele B, Fluieraru S, Teot L (September 2018). "Antibiotic therapy of diabetic foot infections: A systematic review of randomized controlled trials". Wound Repair and Regeneration. 26 (5): 381–391. doi:10.1111/wrr.12649. PMID 30099812. S2CID 51966152.
- "Invanz: EPAR – Summary for the public" (PDF). European Medicines Agency. 2 December 2016.
External links
- "Ertapenem". Drug Information Portal. U.S. National Library of Medicine.