Iodine cycle
The iodine cycle is a biogeochemical cycle that primarily consists of natural[1] and biological processes[3] that exchange iodine through the lithosphere, hydrosphere, and atmosphere.[3][2] Iodine exists in many forms, but in the environment, it generally has an oxidation state of -1, 0, or +5.[1]
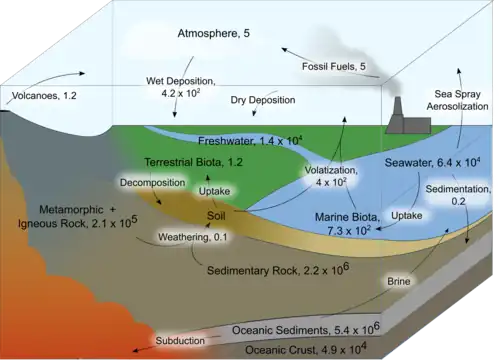
Oceanic cycling
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
|
Iodine in the ocean exists mostly in oceanic sediments and seawater.[4] During subduction of oceanic crust and seawater, most of the iodine cycles into seawater through brine, while a minor amount is cycled into the mantle.[4] Marine biota, including seaweed and fish, accumulate iodine from the seawater and return it during decomposition.[2] Sedimentation of oceanic iodine replenishes the ocean sediment sink. [1]
The losses of iodine from the oceanic sink are to the atmospheric sink.[1] Sea spray aerosolization accounts for a portion of this loss.[2] However, the majority of the iodine cycled into the atmosphere occurs through biological conversion of iodide and iodate to methyl forms, primarily methyl iodide.[3] Algae, phytoplankton, and bacteria are involved in reducing the stable Iodate ion to iodide,[5] and different species produce volatile methyl iodide which leaves the oceans and forms aerosols in the atmosphere.[3]
Terrestrial cycling
Iodine rarely occurs naturally in mineral form, so it comprises a very small portion of rocks by mass.[2] Sedimentary rocks have higher concentrations of iodine compared to metamorphic and igneous rocks.[4] Due to the low concentration of iodine in rocks, weathering is a minor flux of iodine to soils and the freshwater hydrosphere.[1]
Soils contain a much higher concentration of iodine compared to their parent rock, though most of it is bound to organic and inorganic matter, potentially due to microbial activity.[4] The major source of iodine to soils is through dry and wet deposition of aerosolized iodine in the atmosphere.[1] Due to the high production of atmospheric iodine from the oceans, both the concentration of iodine and the flux of iodine to soils is greatest near coastal regions.[1] Plants uptake iodine from the soil through their roots and return the iodine when they decompose.[2] Fauna that consume plants may uptake this iodine but similarly return it to soils upon decomposition.[2] Some iodine may also be cycled into the freshwater hydrosphere through leaching and runoff, where it may return to the oceans.[1]
Similar to oceanic iodine, the majority of iodine cycled out of soil is volatilized through conversion to methyl forms of iodine by bacteria.[3] Unlike ocean volatilization, however, bacteria are thought to be the only organisms responsible for volatilization in soils.[4]
Anthropogenic influences
Iodine is a necessary trace nutrient for human health and is used as a product for various industries.[3] Iodine intended for human use and consumption is taken from brines, which accounts for a minor perturbation to the global iodine cycle.[1] A much larger anthropogenic impact is through the burning of fossil fuels, which releases iodine into the atmosphere.[1]
Iodine-129, a radioisotope of iodine, is a waste product of nuclear power generation and weapons testing.[3] Unless present in high concentrations, I-192 likely does not present danger to human health.[6] Early research has attempted to use the I-129/I-127 ratio as a tracer for the iodine cycle.[6]
References
- Fuge, Ronald; Johnson, Christopher C. (1986). "The geochemistry of iodine — a review". Environmental Geochemistry and Health. 8 (2): 31–54. doi:10.1007/BF02311063. ISSN 1573-2983. PMID 24213950. S2CID 45457666.
- Whitehead, D. C. (1984). "The distribution and transformations of iodine in the environment". Environment International. 10 (4): 321–339. doi:10.1016/0160-4120(84)90139-9. ISSN 0160-4120.
- Amachi, Seigo (2008). "Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine". Microbes and Environments. 23 (4): 269–276. doi:10.1264/jsme2.ME08548. ISSN 1342-6311. PMID 21558718.
- Muramatsu, Yasuyuki; Yoshida, Satoshi; Fehn, Udo; Amachi, Seigo; Ohmomo, Yoichiro (2004). "Studies with natural and anthropogenic iodine isotopes: iodine distribution and cycling in the global environment". Journal of Environmental Radioactivity. Papers from the International Conference on Radioactivity in the Environment, Monaco, 1-5 September 2002. 74 (1): 221–232. doi:10.1016/j.jenvrad.2004.01.011. ISSN 0265-931X. PMID 15063550.
- Reyes-Umana, Victor; Henning, Zachary; Lee, Kristina; Barnum, Tyler P.; Coates, John D. (2021-07-02). "Genetic and phylogenetic analysis of dissimilatory iodate-reducing bacteria identifies potential niches across the world's oceans". The ISME Journal. 16 (1): 38–49. doi:10.1038/s41396-021-01034-5. ISSN 1751-7370. PMC 8692401. PMID 34215855.
- Hou, Xiaolin; Hansen, Violeta; Aldahan, Ala; Possnert, Göran; Lind, Ole Christian; Lujaniene, Galina (2009). "A review on speciation of iodine-129 in the environmental and biological samples". Analytica Chimica Acta. 632 (2): 181–196. doi:10.1016/j.aca.2008.11.013. ISSN 0003-2670. PMID 19110092. S2CID 11740112.