Pirtobrutinib
Pirtobrutinib, sold under the brand name Jaypirca, is an anticancer medication that is used to treat mantle cell lymphoma.[2] It inhibits B cell lymphocyte proliferation and survival by binding and inhibiting Bruton's tyrosine kinase (BTK).[3] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Jaypirca |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
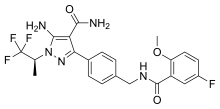
| Formula | C22H21F4N5O3 |
| Molar mass | 479.436 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The most common adverse reactions include fatigue, musculoskeletal pain, diarrhea, edema, dyspnea, pneumonia, and bruising.[2] Grade 3 or 4 laboratory abnormalities include decreased neutrophil counts, lymphocyte counts, and platelet counts.[2]
Pirtobrutinib was approved for medical use in the United States in January 2023.[2][4]
Medical uses
In the US, pirtobrutinib is indicated to treat relapsed or refractory mantle cell lymphoma after at least two lines of systemic therapy, including a Bruton's tyrosine kinase (BTK) inhibitor.[1]
Mechanism of action
B cells are white cells of the lymphocyte subtype that produce antibodies, but when some of them grow uncontrollably they can be a cause of cancer. A key enzyme in B cell stimulation and survival is BTK, and pirtobrutinib inhibits BTK in a way that is different from the prototypical BTK inhibitor ibrutinib by binding in a different way that avoids a genetic change (mutation at active site cysteine residue C481 in BTK) that can make some tumors less responsive to ibrutinib.[3]
History
Pirtobrutinib is manufactured by Eli Lilly and Company and was approved by the US Food and Drug Administration in January 2023, for the treatment of mantle cell lymphoma that has become refractory to other BTK inhibitors.[5]
Efficacy was evaluated in BRUIN (NCT03740529), an open-label, multicenter, single-arm trial of pirtobrutinib monotherapy that included 120 participants with MCL previously treated with a BTK inhibitor.[2] Participants had a median of three prior lines of therapy, with 93% having two or more prior lines.[2] The most common prior BTK inhibitors received were ibrutinib (67%), acalabrutinib (30%), and zanubrutinib (8%); 83% had discontinued their last BTK inhibitor due to refractory or progressive disease.[2]
Society and culture
Legal status
On 26 April 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Jaypirca, intended for the treatment of relapsed or refractory mantle cell lymphoma (MCL).[6] The applicant for this medicinal product is Eli Lilly Nederland B.V.[6]
References
- "Jaypirca- pirtobrutinib tablet, coated". DailyMed. 27 January 2023. Archived from the original on 11 February 2023. Retrieved 11 February 2023.
- "FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma". FDA. 2023-01-27. Archived from the original on 2023-01-28. Retrieved 2023-01-28.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Aslan B, Kismali G, Iles LR, Manyam GC, Ayres ML, Chen LS, et al. (May 2022). "Pirtobrutinib inhibits wild-type and mutant Bruton's tyrosine kinase-mediated signaling in chronic lymphocytic leukemia". Blood Cancer Journal. 12 (5): 80. doi:10.1038/s41408-022-00675-9. ISSN 2044-5385. PMC 9123190. PMID 35595730.
- "U.S. FDA Approves Jaypirca (pirtobrutinib), the First and Only Non-Covalent (Reversible) BTK Inhibitor, for Adult Patients with Relapsed or Refractory Mantle Cell Lymphoma After at Least Two Lines of Systemic Therapy, Including a BTK Inhibitor" (Press release). Eli Lilly. 27 January 2023. Archived from the original on 30 January 2023. Retrieved 31 January 2023 – via PR Newswire.
- "FDA approves Eli Lilly's drug for rare blood cancer". Reuters. 27 January 2023. Archived from the original on 2023-01-28.
- "Jaypirca: Pending EC decision". European Medicines Agency. 26 April 2023. Retrieved 27 April 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading
- Cohen JB, Shah NN, Alencar AJ, Gerson JN, Patel MR, Fakhri B, et al. (October 2022). "MCL-133 Pirtobrutinib, a Highly Selective, Non-Covalent (Reversible) BTK Inhibitor in Previously Treated Mantle Cell Lymphoma: Updated Results From the Phase 1/2 BRUIN Study". Clinical Lymphoma, Myeloma & Leukemia. 22 (Suppl 2): S394–S395. doi:10.1016/S2152-2650(22)01569-5. PMID 36164120.
- Eyre TA, Shah NN, Dreyling M, Jurczak W, Wang Y, Cheah CY, et al. (November 2022). "BRUIN MCL-321: phase III study of pirtobrutinib versus investigator choice of BTK inhibitor in BTK inhibitor naïve mantle cell lymphoma". Future Oncology. 18 (36): 3961–3969. doi:10.2217/fon-2022-0976. PMID 36377973.
- Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. (March 2021). "Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study". Lancet. 397 (10277): 892–901. doi:10.1016/S0140-6736(21)00224-5. PMID 33676628. S2CID 232116910.