Mechanical filter (respirator)
Mechanical filters are a class of filter for air-purifying respirators that mechanically stops particulates from reaching the wearer's nose and mouth. They come in multiple physical forms.
.jpg.webp)

_(cropped).jpg.webp)
Mechanism of operation

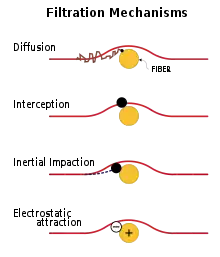
Mechanical filter respirators retain particulate matter such as dust created during woodworking or metal processing, when contaminated air is passed through the filter material. Wool is still used today as a filter, along with plastic, glass, cellulose, and combinations of two or more of these materials. Since the filters cannot be cleaned and reused and have a limited lifespan, cost and disposability are key factors. Single-use, disposable and replaceable-cartridge models exist.[3]
Mechanical filters remove contaminants from air in the following ways:
- by interception when particles following a line of flow in the airstream come within one radius of a fiber and adhere to it;[3]
- by impaction, when larger particles unable to follow the curving contours of the airstream are forced to embed in one of the fibers directly; this increases with diminishing fiber separation and higher air flow velocity[3]
- by an enhancing mechanism called diffusion, where gas molecules collide with the smallest particles, especially those below 100 nm in diameter, which are thereby impeded and delayed in their path through the filter; this effect is similar to Brownian motion and increases the probability that particles will be stopped by either of the two mechanisms above; it becomes dominant at lower air flow velocities[3]
- by using electret filter material (usually, electrospun plastic fibers) to attract or repel particles with an electrostatic charge, so that they are more likely to collide with the filter surface
- by using certain coatings on the fibers that kill or deactivate infectious particles colliding with them (such as salt)[4]
- by using gravity and allowing particles to settle into the filter material (this effect is typically negligible); and[5]
- by using the particles themselves, after the filter has been used, to act as a filter medium for other particles.
Considering only particulates carried on an air stream and a fiber mesh filter, diffusion predominates below the 0.1 μm diameter particle size. Impaction and interception predominate above 0.4 μm. In between, near the most penetrating particle size of 0.3 μm, diffusion and interception predominate.[3]
For maximum efficiency of particle removal and to decrease resistance to airflow through the filter, particulate filters are designed to keep the velocity of air flow through the filter as low as possible. This is achieved by manipulating the slope and shape of the filter to provide larger surface area.
High-efficiency particulate air (HEPA)filters are all filters meeting certain efficiency standards. A HEPA filter must remove at least 99.97% (US) or 99.95% (EU) of all airborne particulates with aerodynamic diameter of 0.3 μm. Particles both smaller and larger are easier to catch, and thus removed with a higher efficiency.[6][7][8] People often assume that particles smaller than 0.3 microns would be more difficult to filter efficiently; however, the physics of Brownian motion at such smaller sizes boosts filter efficiency (see figure).[2]

Materials
Mechanical filters can be made of a fine mesh of synthetic polymer fibers.[9][10] The fibers are produced by melt blowing.[11] The fibers are charged as they are blown to produce an electret,[12] and then layered to form a nonwoven polypropylene fabric.[9][10]
Exhalation valves

Some masks have check valves,[13] that let the exhaled air go out unfiltered. The certification grade of the mask (as N95 or FFP2) is about the mask itself and it does not warrant any safety about the air that is expelled by the wearer through the valve. A mask with valve will reduce inwards leakages, thus improving the wearer protection.[13]
Unfiltered-exhalation valves are sometimes found in both filtering facepiece[13] and elastomeric respirators;[14] PAPRs cannot by nature ever filter exhaled air.[15] As a result, these masks are believed to be incapable of source control, which is protecting others against an infection in the wearer's breath.[14] They are not generally designed for healthcare use, as of 2017.[16] Despite the aforementioned belief, a 2020 research by the NIOSH and CDC shows that an uncovered exhalation valve already provides source control on a level similar to, or even better than, surgical masks.[17]
During the COVID-19 pandemic, masks with unfiltered-exhalation valves did not meet the requirements of some mandatory mask orders.[18][19] It is possible to seal some unfiltered exhalation valves[20] or to cover it with an additional surgical mask; this might be done where mask shortages make it necessary.[21][22]
Uses
Filtering facepiece respirators
Filtering facepiece respirator (FFPs) are disposable face masks produced from a whole piece of filtering material. FFPs (such as N95 masks) are discarded when they become unsuitable for further use due to considerations of hygiene, excessive resistance, or physical damage.[23]
Mass production of filtering facepieces started in 1956. The air was purified with nonwoven filtering material consisting of polymeric fibers carrying a strong electrostatic charge. Respirator was used in nuclear industry, and then in other branches of economy. For ~60 years, more than 6 billion respirators were manufactured.[24] Unfortunately, the developers overestimated the efficiency (APF 200-1000 compared to the modern value of 10–20), which led to serious errors in the choice of personal protective equipment by employers.
Elastomeric respirators

Elastomeric respirators are reusable devices with exchangeable cartridge filters that offer comparable protection to N95 masks.[25] The filters must be replaced when soiled, contaminated, or clogged.[14]
They may have exhalation valves. Full-face versions of elastomeric respirators seal better and protect the eyes. Fitting and inspection is essential to effectiveness.[14]
Powered air-purifying respirators (PAPRs)
PAPRs are masks with an electricity-powered blower that blows air through a filter to the wearer. Because they create positive pressure, they need not be tightly fitted.[26] PAPRs typically do not filter exhaust from the wearer.[27]
Shortcomings
The electrostatic filters in respirators are much easier to breathe through than cloth masks, however, when respirators are worn with additional coverings, such as surgical mask material, then they can make breathing harder for the wearer. As a result, exposure to carbon dioxide may exceed its OELs (0.5% by volume for 8-hour shift; 1.4% for 15 minutes exposure[28]), with CO2 levels inside reaching up to 2.6% for elastomeric respirators and up to 3.5 for FFRs. Mean values for several models; some models may provide a stronger exposure to carbon dioxide. These values are comparable to the CO2 levels that normally occur within the trachea, and the volume inside a respirator facepiece is a fraction of the total volume inhaled with each breath, so the total CO2 concentration for each breath is much less than the concentration within the small volume of the facepiece itself.[lower-alpha 1][29][30][31] Skin irritation and acne (from humidity and skin contact) can be an annoyance.[32] The UK HSE textbook recommends limiting the use of respirators without air supply to 1 hour,[33] while OSHA recommends respirator use for up to eight hours.
Almost all filtration methods perform poorly outside when environmental airborne water levels are high, causing saturation and clogging, increasing breathing resistance, and the collection of water on the electrostatic filter fibers can reduce the efficiency of the filter. Bidirectional air flow (as used on masks without an exhalation valve) compounds this problem further. Design standards are typically used for 'indoor' settings only.
Filtration standards
U.S. standards (N95 and others)
In the United States, the National Institute for Occupational Safety and Health defines the following categories of particulate filters according to their NIOSH air filtration rating.[34] (Categories highlighted in blue have not actually been applied to any products.)
| Oil resistance | Rating | Description |
|---|---|---|
| Not oil resistant | N95 | Filters at least 95% of airborne particles |
| N99 | Filters at least 99% of airborne particles | |
| N100 | Filters at least 99.97% of airborne particles | |
| Oil resistant | R95 | Filters at least 95% of airborne particles |
| R99 | Filters at least 99% of airborne particles | |
| R100 | Filters at least 99.97% of airborne particles | |
| Oil proof | P95 | Filters at least 95% of airborne particles |
| P99 | Filters at least 99% of airborne particles | |
| P100 | Filters at least 99.97% of airborne particles |
Additionally, HE (high-efficiency) filters are the class of particulate filter used with powered air-purifying respirators. These are 99.97% efficient against 0.3 micron particles, the same as a P100 filter.[35][36][37]
During the COVID-19 pandemic, the US Occupational Safety and Health Administration issued an equivalency table, giving similar foreign standards for each US standard.[38]
In the United States, N95 respirators are designed and/or made by companies such as 3M, Honeywell, Cardinal Health, Moldex,[39] Kimberly-Clark, Alpha Pro Tech,[40] Gerson,[41] Prestige Ameritech and Halyard Health. In Canada, N95s are made by AMD Medicom,[42] Vitacore,[43] Advanced Material Supply,[44] Eternity[45] and Mansfield Medical.[46] The Taiwanese company Makrite makes N95s as well as similar respirators for a number of other countries.[47] Degil is a label for some of Makrite's respirators.
European standards (FFP2 and others)

.jpg.webp)
European standard EN 143 defines the 'P' classes of particle filters that can be attached to a face mask, and European standard EN 149 defines the following classes of "filtering half masks" or "filtering facepieces" (FFP), that is respirators that are entirely or substantially constructed of filtering material:[48]
| Standard | Class | Filter type | Filter penetration limit (at 95 L/min air flow) | Inward leakage | Typical elastic band |
|---|---|---|---|---|---|
| EN 149 | FFP1 | Mask | Filters at least 80% of airborne particles | <22% | yellow |
| FFP2 | Filters at least 94% of airborne particles | <8% | blue or white | ||
| FFP3 | Filters at least 99% of airborne particles | <2% | red | ||
| EN 143 | P1 | Attachment | Filters at least 80% of airborne particles | N/A | N/A |
| P2 | Filters at least 94% of airborne particles | ||||
| P3 | Filters at least 99.95% of airborne particles | ||||
| EN 14683[49] | Type I | Mask | Less than 98% droplet filtration, intended for use by patients | N/A | N/A |
| Type II | Not fluid-resistant, 98% droplet filtration, intended for use by healthcare workers in droplet-free environments | ||||
| Type IIR | Fluid-resistant, 98% droplet filtration, surgical |
Both European standard EN 143 and EN 149 test filter penetration with dry sodium chloride and paraffin oil aerosols after storing the filters at 70 °C (158 °F) and −30 °C (−22 °F) for 24 h each. The standards include testing mechanical strength, breathing resistance and clogging. EN 149 tests the inward leakage between the mask and face, where 10 human subjects perform 5 exercises each. The truncated mean of average leakage from 8 individuals must not exceed the aforementioned values.[50]: § 8.5
In Germany, FFP2 respirators are made by companies such as Dräger, Uvex[51] and Core Medical.[52] In Belgium, Ansell[53] makes FFP2 masks. In France, the company Valmy[54] makes them. In the United Kingdom, the company Hardshell[55] has recently begun making FFP2 masks.
Other standards (KN95 and others)

.jpg.webp)
Respirator standards around the world loosely fall into the two camps of US- and EU-like grades. According to 3M, respirators made according to the following standards are equivalent to US N95 or European FFP2 respirators "for filtering non-oil-based particles such as those resulting from wildfires, PM 2.5 air pollution, volcanic eruptions, or bioaerosols (e.g. viruses)":[56]
- Chinese KN95 (GB2626-2006): similar to US. Has category KN (non-oily particles) and KP (oily particles), 90/95/100 versions. EU-style leakage requirements.[57][58] In China, KN95 respirators are made by companies such as Guangzhou Harley,[59] Guangzhou Powecom,[60] Shanghai Dasheng[61] and FLTR.[62]
- Korean 1st Class (KMOEL - 2017–64), also referred to as "KF94": EU grades, KF 80/94/99 for second/first/special.[63] In Korea, KF94 respirators are made by companies such as LG, Soomlab,[64] Airqueen,[65] Kleannara,[66] Dr. Puri,[67] Bluna[68] and BOTN.[69][70] The Hong Kong company Masklab also makes KF-style respirators.[71]
- Australian/New Zealand P2 (AS/NZ 1716:2012): similar to EU grades.
The NPPTL has also published a guideline for using non-NIOSH masks instead of the N95 in the COVID-19 response. The OSHA has a similar document. The following respirator standards are considered similar to N95 in the US:[72][73]
- Japanese DS2/RS2 (JMHLW-Notification 214, 2018): EU-like grades with two-letter prefix – first letter D/R stands for disposable or replaceable; second letter S/L stands for dry (NaCl) or oily (DOP oil) particles.[5] Japanese DS2 respirators are made by companies such as Hogy Medical,[74] Koken,[75] Shigematsu,[76] Toyo Safety,[77] Trusco,[78] Vilene[79] and Yamamoto Safety.[80]
- Mexican N95 (NOM-116-2009): same grades as in NIOSH.
- Brazilian PFF2 (ABNT/NBR 13698:2011): EU-like grades.
Disinfection and reuse
Hard filtering facepiece respirator masks are generally designed to be disposable, for 8 hours of continuous or intermittent use. One laboratory found that there was a decrease in fit quality after five consecutive donnings.[13] Once they are physically too clogged to breathe through, they must be replaced.
Hard filtering facepiece respirator masks are sometimes reused, especially during pandemics, when there are shortages. Infectious particles could survive on the masks for up to 24 hours after the end of use, according to studies using models of SARS-CoV-2;[13] In the COVID-19 pandemic, the US CDC recommended that if masks run short, each health care worker should be issued with five masks, one to be used per day, such that each mask spends at least five days stored in a paper bag between each use. If there are not enough masks to do this, they recommend sterilizing the masks between uses.[81] Some hospitals have been stockpiling used masks as a precaution.[82] The US CDC issued guidelines on stretching N95 supplies, recommending extended use over re-use. They highlighted the risk of infection from touching the contaminated outer surface of the mask, which even professionals frequently unintentionally do, and recommended washing hands every time before touching the mask. To reduce mask surface contamination, they recommended face shields, and asking patients to wear masks too ("source masking").[83]
Apart from time, other methods of disinfection have been tested. Physical damage to the masks has been observed when microwaving them, microwaving them in a steam bag, letting them sit in moist heat, and hitting them with excessively high doses of ultraviolet germicidal irradiation (UVGI). Chlorine-based methods, such as chlorine bleach, may cause residual smell, offgassing of chlorine when the mask becomes moist, and in one study, physical breakdown of the nosepads, causing increased leakage.[13] Fit and comfort do not seem to be harmed by UVGI, moist heat incubation, and microwave-generated steam.[13]
Some methods may not visibly damage the mask, but they ruin the mask's ability to filter. This has been seen in attempts to sterilize by soaking in soap and water, heating dry to 160 °C (320 °F), and treating with 70% isopropyl alcohol, and hydrogen peroxide gas plasma[13] (made under a vacuum with radio waves[84]). The static electrical charge on the microfibers (which attracts or repels particles passing through the mask, making them more likely to move sideways and hit and stick to a fiber; see electret) is destroyed by some cleaning methods. UVGI (ultraviolet light), boiling water vapour, and dry oven heating do not seem to reduce the filter efficiency, and these methods successfully decontaminate masks.[13]
UVGI (an ultraviolet method), ethylene oxide, dry oven heating and vaporized hydrogen peroxide are currently the most-favoured methods in use in hospitals, but none have been properly tested.[13] Where enough masks are available, cycling them and reusing a mask only after letting it sit unused for five days is preferred.[81]
It has been shown[85] that masks can also be sterilized by ionizing radiation. Gamma radiation and high energy electrons penetrate deeply into the material and can be used to sterilize large batches of masks within a short time period. The masks can be sterilized up to two times but have to be recharged after every sterilization as the surface charge is lost upon radiation.
A recent development is a composite fabric that can deactivate both biological and chemical threats [86]
Notes
- For example, IDLH for CO2 = 4%, but filtering facepiece "AOSafety Pleats Plus" provided concentration up to 5.8%.
References
- "Elastomeric Respirators: Strategies During Conventional and Surge Demand Situations". U.S. Centers for Disease Control and Prevention. 11 February 2020.
- Wei, Neo Kang (6 May 2019). "What is PM0.3 and Why Is It Important?". Smart Air Filters.
- TSI Incorporated. "Mechanisms of Filtration for High Efficiency Fibrous Filters - Application Note ITI-041" (PDF). Retrieved 29 April 2020.
- Quan, Fu-Shi; Rubino, Ilaria; Lee, Su-Hwa; Koch, Brendan; Choi, Hyo-Jick (2017-01-04). "Universal and reusable virus deactivation system for respiratory protection". Scientific Reports. 7 (1): 39956. Bibcode:2017NatSR...739956Q. doi:10.1038/srep39956. ISSN 2045-2322. PMC 5209731. PMID 28051158.
- "Standard for Dust Mask". JICOSH Home.
- Guidance for Filtration and Air-Cleaning Systems to Protect Building Environments from Airborne Chemical, Biological, or Radiological Attacks (PDF). Cincinnati, OH: National Institute for Occupational Safety and Health. April 2003. pp. 8–12. doi:10.26616/NIOSHPUB2003136. Retrieved February 9, 2020.
- Japuntich, Daniel A.; Franklin, Luke M.; Pui, David Y.; Kuehn, Thomas H.; Kim, Seong Chan; Viner, Andrew S. (2006-11-10). "A comparison of two nano-sized particle air filtration tests in the diameter range of 10 to 400 nanometers". Journal of Nanoparticle Research. 9: 93–107. doi:10.1007/s11051-006-9179-1. S2CID 136771817.
- Perry, J. L.; Agui, J. H.; Vijayakimar, R (2016-05-01). "Submicron and Nanoparticulate Matter Removal by HEPA-Rated Media Filters and Packed Beds of Granular Materials". NASA.
- Xie, John (2020-03-19). "World Depends on China for Face Masks But Can Country Deliver?". Voice of America.
- Evan, Melanie; Hufford, Austen (March 7, 2020). "Critical Component of Protective Masks in Short Supply - The epidemic has driven up demand for material in N95 filters; 'everyone thinks there is this magic factory somewhere'". The Wall Street Journal.
- Feng, Emily (2020-03-16). "COVID-19 Has Caused A Shortage Of Face Masks. But They're Surprisingly Hard To Make". NPR.
- US 4215682A
- Godoy, Laura R. Garcia; Jones, Amy E.; Anderson, Taylor N.; Fisher, Cameron L.; Seeley, Kylie M. L.; Beeson, Erynn A.; Zane, Hannah K.; Peterson, Jaime W.; Sullivan, Peter D. (1 May 2020). "Facial protection for healthcare workers during pandemics: a scoping review". BMJ Global Health. 5 (5): e002553. doi:10.1136/bmjgh-2020-002553. ISSN 2059-7908. PMC 7228486. PMID 32371574.
- "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020.
- Institute of Medicine (2015). "Defining PAPRs and Current Standards". The Use and Effectiveness of Powered Air Purifying Respirators in Health Care: Workshop Summary. Washington, D.C.: National Academies Press. doi:10.17226/18990. ISBN 978-0-309-31595-1. PMID 25996018.
- Radonovich, Lew (5 September 2017). "Elastomeric and Powered-Air Purifying Respirators in U.S. Healthcare" (PDF).
- Portnoff L, Schall J, Brannen J, Suhon N, Strickland K, Meyers J (2020). "Filtering Facepiece Respirators with an Exhalation Valve: Measurements of Filtration Efficiency to Evaluate Their Potential for Source Control". DHHS (NIOSH) Publication No. 2021-107. National Institute for Occupational Safety and Health. doi:10.26616/NIOSHPUB2021107.
- Wilson, Mark (April 28, 2020). "What is a mask valve, and why are cities banning them?". MSN.
- Webeck, Evan (22 April 2020). "Coronavirus: Bay Area mask order takes effect Wednesday. Here's what you need to know". The Mercury News.
- Filtering Facepiece Respirators with an Exhalation Valve: Measurements of Filtration Efficiency to Evaluate Their Potential for Source Control (Technical report). 30 June 2021. doi:10.26616/NIOSHPUB2021107. S2CID 235456824.
- Liu, DCY; Koo, TH; Wong, JKK; Wong, YH; Fung, KSC; Chan, Y; Lim, HS (August 2020). "Adapting re-usable elastomeric respirators to utilise anaesthesia circuit filters using a 3D-printed adaptor - a potential alternative to address N95 shortages during the COVID-19 pandemic". Anaesthesia. 75 (8): 1022–1027. doi:10.1111/anae.15108. PMC 7267584. PMID 32348561.
- "San Antonio hospital could have an answer to the PPE crisis-- elastomeric masks". kens5.com. May 1, 2020.
But she added you can easily cover the mask with a surgical mask or shield.
- "Respirator Trusted-Source Information: What are they?". U.S. National Institute for Occupational Safety and Health. 2018-01-29. Retrieved 2020-03-27.
- Petryanov, Igor (2015). "Chapters 3 & 5". Лепесток. Лёгкие респираторы [Lepestok. Filtering facepiece respirators] (in Russian) (2nd ed.). Moscow: Science. p. 320. ISBN 978-5-02-039145-1.
- Bach, Michael (6 July 2017). "Understanding respiratory protection options in Healthcare: The Overlooked Elastomeric". NIOSH Science Blog. CDC.
- Boškoski, Ivo; Gallo, Camilla; Wallace, Michael B.; Costamagna, Guido (27 April 2020). "COVID-19 pandemic and personal protective equipment shortage: protective efficacy comparing masks and scientific methods for respirator reuse". Gastrointestinal Endoscopy. 92 (3): 519–523. doi:10.1016/j.gie.2020.04.048. ISSN 0016-5107. PMC 7184993.
- "Defining PAPRs and Current Standards". The Use and Effectiveness of Powered Air Purifying Respirators in Health Care: Workshop Summary. 2. Defining PAPRs and Current Standards. 7 May 2015.
{{cite book}}:|work=ignored (help) - Popova, Anna, ed. (2018). "Substance #2138 Carbon dioxide". Hygienic standard 2.2.5.3532-18.Occupational exposure limits for toxic substances in workplace air [ГН 2.2.5.3532-18 Предельно допустимые концентрации (ПДК) вредных веществ в воздухе рабочей зоны] (in Russian). Moscow: Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing. p. 170.
- Sinkule, Edward James; Powell, Jeffrey Bryon; Goss, Fredric Lee (April 2013). "Evaluation of N95 respirator use with a surgical mask cover: effects on breathing resistance and inhaled carbon dioxide". The Annals of Occupational Hygiene. 57 (3): 384–398. doi:10.1093/annhyg/mes068. ISSN 1475-3162. PMID 23108786. S2CID 22915157.
- Roberge, Raymond J.; Coca, Aitor; Williams, W. Jon; Powell, Jeffrey B.; Palmiero, Andrew J. (May 2010). "Physiological impact of the N95 filtering facepiece respirator on healthcare workers". Respiratory Care. 55 (5): 569–577. ISSN 0020-1324. PMID 20420727.
- Sinkule E, Turner N, Hota S (2003). "Automated breathing and metabolic simulator (ABMS) CO2 test for powered and non-powered air-purifying respirators, airline respirators, and gas mask.". American Industrial Hygiene Conference and Exposition. American Industrial Hygiene Association. doi:10.3320/1.2757905.
- Chris C.I. Foo, Anthony T.J. Goon, Yung-Hian Leow, Chee-Leok Goh (2006). "Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome – a descriptive study in Singapore". Contact Dermatitis. John Wiley & Sons. 55 (5): 291–294. doi:10.1111/j.1600-0536.2006.00953.x. ISSN 0105-1873. PMC 7162267. PMID 17026695.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - The Health and Safety Executive (2013). Respiratory protective equipment at work. A practical guide. HSG53 (4 ed.). Crown. p. 59. ISBN 978-0-71766-454-2. Retrieved 10 June 2018.
- Metzler, R; Szalajda, J (2011). "NIOSH Fact Sheet: NIOSH Approval Labels - Key Information to Protect Yourself" (PDF). DHHS (NIOSH) Publication No. 2011-179. ISSN 0343-6993.
- "Considerations for Optimizing the Supply of Powered Air-Purifying Respirators (PAPRs)". U.S. Centers for Disease Control and Prevention. 2020-04-19. Retrieved 2020-05-25.
- Vanessa, Roberts (Fall 2014). "To PAPR or Not to PAPR?". Canadian Journal of Respiratory Therapy. 50 (3): 87–90. PMC 4456839. PMID 26078617.
- "Understanding Respiratory Protection Against SARS". U.S. National Institute for Occupational Safety and Health. 2020-04-09. Retrieved 2020-05-26.
- "Enforcement Guidance for Use of Respiratory Protection Equipment Certified under Standards of Other Countries or Jurisdictions During the Coronavirus Disease 2019 (COVID-19) Pandemic". www.osha.gov. [US] Occupational Safety and Health Administration. Retrieved 20 August 2020.
- "Cool & Comfortable PPE | Disposable Respirators & Masks". Moldex.
- "StackPath".
- "1730 N95 Particulate Respirator Made in USA".
- "SafeMask® Cone Mask".
- "Vitacore Industries Inc". www.vitacore.ca.
- https://advanced-material-supply.myshopify.com/collections/n95-masks
- "ECAN95 95PFE – Eternity". eternitymsm.com. Archived from the original on 2020-12-23.
- "PRODUCTS – Mansfield Medical".
- "Products – Makrite".
- "Fiche pratique de sécurité ED 105. Appareils de protection respiratoire et métiers de la santé" (PDF). inrs.fr. INRS. Retrieved 7 April 2020.
- "COVID-19 Technical Specifications for Personal Protective Equipment and Related IPC supplies" (PDF). World Health Organization.
- "NF EN 149+A1". www.boutique.afnor.org. September 2009. alternative source
- "Dust Masks | Construction Face Mask". uvex safety.
- "FFP2 Masken - CORE Medical Supply GMBH".
- "SANDEL® Respiratory face masks".
- "VALMY". www.valmy.eu.
- "FFP2 FACE MASK". hardshell.co.uk.
- "Technical Bulletin: Comparison of FFP2, KN95, and N95 and Other Filtering Facepiece Respirator Classes" (PDF). 3M Personal Safety Division. January 2020.
- "China Releases an Updated Mandatory Standard GB 2626-2019 Respiratory Protection - Non-Powered Air-Purifying Particle Respirator". HKTDC Research. 20 April 2020. Classification and Marking: 2. Filter element categorization. Retrieved 27 July 2020.
- "China Mandatory Standard GB 2626-2019 Respiratory Protection—Non-Powered Air-Purifying Particle Respirator Effective Date Postponed to July 1 2021" (PDF). Bureau Veritas. 24 June 2020. Retrieved 27 July 2020.
- "建站成功". harleykn95.com.
- "Powecom - home page". www.powecom.com.
- "Dasheng Health Products Manufacturing". www.dashengmask.com.
- "FLTR95 Sealing Face Masks 100PK - White".
- Jung, Hyejung; Kim, Jongbo; Lee, Seungju; Lee, Jinho; Kim, Jooyoun; Tsai, Perngjy; Yoon, Chungsik (2014). "Comparison of Filtration Efficiency and Pressure Drop in Anti-Yellow Sand Masks, Quarantine Masks, Medical Masks, General Masks, and Handkerchiefs". Aerosol and Air Quality Research. 14 (3): 991–1002. doi:10.4209/aaqr.2013.06.0201.
- "[Global] Soomlab Mask".
- "HOME". AirQUEEN™ Nano Mask.
- "Main products".
- "DR. PURI KF94 MASK | DR PURI KF94 FACE MASK MADE IN KOREA". drpurimask.com. Archived from the original on 2020-11-01.
- "블루나(BLUNA) 프리미엄 아기물티슈". 블루나(BLUNA) 프리미엄 아기물티슈.
- "TIA - BOTN KF94". en.botn.co.kr.
- "Aaron Collins - YouTube". www.youtube.com.
- "KF Series".
- "NPPTL Respirator Assessments to Support the COVID-19 Response, International Respirator Assessment Request". NPPTL | NIOSH | CDC. 24 April 2020.
- "Enforcement Guidance for Use of Respiratory Protection Equipment Certified under Standards of Other Countries or Jurisdictions During the Coronavirus Disease 2019 (COVID-19) Pandemic". Occupational Safety and Health Administration.
- "株式会社ホギメディカル".
- "クリーン、ヘルス、セーフティで社会に 興研株式会社". www.koken-ltd.co.jp.
- "使い捨て式防じんマスク | 製品情報 | 株式会社 重松製作所".
- "国家検定合格品 - メガネ - 製品情報 | トーヨーセフティー".
- "TRUSCO トラスコ中山株式会社". www.trusco.co.jp.
- "製品情報:メディカル資材 医療用マスク&キャップ". www.vicre.co.jp.
- "使い捨て式マスク,使い捨て式防じんマスク(Ds2) | Yamamoto 公式オンラインショップ | Yamamoto Safety Online Shop".
- "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020.
- Mills, Stu (10 April 2020). "Researchers looking at innovative ways to sterilize single-use masks". Canadian Broadcasting Corporation.
- "Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings". cdc.gov. NIOSH Workplace Safety and Health Topic. CDC. 27 March 2020.
- "Hydrogen Peroxide Gas Plasma". cdc.gov. Disinfection & Sterilization Guidelines, Guidelines Library, Infection Control. 2019-04-04.
- Pirker, Luka; Krajnc, Anja Pogačnik; Malec, Jan; Radulović, Vladimir; Gradišek, Anton; Jelen, Andreja; Remškar, Maja; Mekjavić, Igor B.; Kovač, Janez; Mozetič, Miran; Snoj, Luka (2020-10-01). "Sterilization of polypropylene membranes of facepiece respirators by ionizing radiation". Journal of Membrane Science. 619: 118756. doi:10.1016/j.memsci.2020.118756. ISSN 0376-7388. PMC 7528844. PMID 33024349.
- Cheung, Yuk Ha; Ma, Kaikai; van Leeuwen, Hans C.; Wasson, Megan C.; Wang, Xingjie; Idrees, Karam B.; Gong, Wei; Cao, Ran; Mahle, John J.; Islamoglu, Timur; Peterson, Gregory W.; de Koning, Martijn C.; Xin, John H.; Farha, Omar K. (October 13, 2021). "Immobilized Regenerable Active Chlorine within a Zirconium-Based MOF Textile Composite to Eliminate Biological and Chemical Threats". Journal of the American Chemical Society. 143 (40): 16777–16785. doi:10.1021/jacs.1c08576. PMID 34590851. S2CID 238229650 – via ACS Publications.