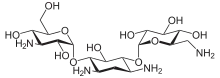
Kanamycin A
Kanamycin A,[2] often referred to simply as kanamycin, is an antibiotic used to treat severe bacterial infections and tuberculosis.[3] It is not a first line treatment.[3] It is used by mouth, injection into a vein, or injection into a muscle.[3] Kanamycin is recommended for short-term use only, usually from 7 to 10 days.[3] As with most antibiotics, it is ineffective in viral infections.[3]
 | |
| Clinical data | |
|---|---|
| Other names | K/KAN/HLK/KM[1] |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, intramuscular |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | very low after by mouth delivery |
| Metabolism | Unknown |
| Elimination half-life | 2 hours 30 minutes |
| Excretion | Urine (as unchanged drug) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.374 |
| Chemical and physical data | |
| Formula | C18H36N4O11 |
| Molar mass | 484.499 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include hearing and balance problems.[3] Kidney problems may also occur.[3] Kanamycin is not recommended during pregnancy as it may harm the baby.[3] It is likely safe during breastfeeding.[4] Kanamycin is in the aminoglycoside family of medications.[3] It works by blocking the production of proteins that are required for bacterial survival.[3]
Kanamycin was first isolated in 1957 by Hamao Umezawa from the bacterium Streptomyces kanamyceticus.[3][5] It was removed from the World Health Organization's List of Essential Medicines in 2019.[6][7] It is no longer marketed in the United States.[3]
Medical uses
Spectrum of activity
Kanamycin is indicated for short-term treatment of bacterial infections caused by one or more of the following pathogens: E. coli, Proteus species (both indole-positive and indole-negative), Enterobacter aerogenes, Klebsiella pneumoniae, Serratia marcescens, and Acinetobacter species. In cases of serious infection when the causative organism is unknown, Kanamycin injection in conjunction with a penicillin- or cephalosporin-type drug may be given initially before obtaining results of susceptibility testing.
Kanamycin does not treat viral infections.[8]
Pregnancy and breastfeeding
Kanamycin is pregnancy category D in the United States.[8]
Kanamycin enters breast milk in small amounts. The manufacturer therefore advises that people should either stop breastfeeding or kanamycin. The American Academy of Pediatrics considers kanamycin okay in breastfeeding.[9]
Children
Kanamycin should be used with caution in newborns due to the risk of increased drug concentration resulting from immature kidney function.[8]
Side effects
Serious side effects include ringing in the ears or loss of hearing, toxicity to kidneys, and allergic reactions to the drug.[10]
Other side effects include:[8]
Gastrointestinal effects
- Nausea, vomiting, diarrhea
Musculoskeletal effects
- Myasthenia gravis
Neurologic effects
- Headache
- Paresthesias
- Blurring of vision
- Neuromuscular blockade
Metabolic effects
- Malabsorption syndrome
Mechanism
Kanamycin works by interfering with protein synthesis. It binds to the 30S subunit of the bacterial ribosome. This results in incorrect alignment with the mRNA and eventually leads to a misread that causes the wrong amino acid to be placed into the peptide. This leads to nonfunctional peptide chains.[11]
Composition
Kanamycin is a mixture of three main components: kanamycin A, B, and C. Kanamycin A is the major component in kanamycin.[12] The effects of these components do not appear to be widely studied as individual compounds when used against prokaryotic and eukaryotic cells.
Biosynthesis
While the main product produced by Streptomyces kanamyceticus is kanamycin A, additional products are also produced, including kanamycin B, kanamycin C, kanamycin D and kanamycin X.
The kanamycin biosynthetic pathway can be divided into two parts. The first part is common to several aminoglycoside antibiotics, such as butirosin and neomycin. In it a unique aminocyclitol, 2-deoxystreptamine, is biosynthesized from D-glucopyranose 6-phosphate in four steps. At this point the kanamycin pathway splits into two branches due to the promiscuity of the next enzyme, which can utilize two different glycosyl donors - UDP-N-acetyl-α-D-glucosamine and UDP-α-D-glucose. One of the branches forms kanamycin C and kanamycin B, while the other branch forms kanamycin D and kanamycin X. However, both kanamycin B and kanamycin D can be converted to kanamycin A, so both branches of the pathway converge at kanamycin A.[13]
Use in research
Kanamycin is used in molecular biology as a selective agent most commonly to isolate bacteria (e.g., E. coli) which have taken up genes (e.g., of plasmids) coupled to a gene coding for kanamycin resistance (primarily Neomycin phosphotransferase II [NPT II/Neo]). Bacteria that have been transformed with a plasmid containing the kanamycin resistance gene are plated on kanamycin (50-100 µg/ml) containing agar plates or are grown in media containing kanamycin (50-100 µg/ml). Only the bacteria that have successfully taken up the kanamycin resistance gene become resistant and will grow under these conditions. As a powder, kanamycin is white to off-white and is soluble in water (50 mg/ml).
At least one such gene, Atwbc19[14] is native to a plant species, of comparatively large size and its coded protein acts in a manner which decreases the possibility of horizontal gene transfer from the plant to bacteria; it may be incapable of giving resistance to bacteria even if gene transfer occurs.
Antibiotic Conjugated Nanoparticle Synthesis
Antibiotic resistance or development of multi-drug resistant bacterial strains is a key challenge for treating bacterial infections. With limited research being carried out to design and develop new antibiotics, novel approaches like functionalizing antibiotic to metal nanoparticles surface to treat resistant bacterial strains have been studied. Kanamycin functionalized gold-nanoparticles (Kan-GNPs) were synthesized and tested for its antibacterial activity against both gram positive and gram negative strains. A dose dependent antibacterial activity was noted for Kan-GNPs in comparison to free kanamycin.[15]
KanMX marker
The selection marker kanMX is a hybrid gene consisting of a bacterial aminoglycoside phosphotransferase (kanr from transposon Tn903) under control of the strong TEF promoter from Ashbya gossypii.[16][17]
Mammalian cells, yeast, and other eukaryotes acquire resistance to geneticin (= G418, an aminoglycoside antibiotic similar to kanamycin) when transformed with a kanMX marker. In yeast, the kanMX marker avoids the requirement of auxotrophic markers. In addition, the kanMX marker renders E. coli resistant to kanamycin. In shuttle vectors the KanMX cassette is used with an additional bacterial promoter. Several versions of the kanMX cassette are in use, e.g. kanMX1-kanMX6. They primarily differ by additional restriction sites and other small changes around the actual open reading frame.[16][18]
References
- "Antibiotic abbreviations list". Retrieved 22 June 2023.
- J. Elks; C. R. Ganellin (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 717–. doi:10.1007/BF00171763. S2CID 122125855.
- "Kanamycin Sulfate". The American Society of Health-System Pharmacists. Archived from the original on 10 September 2017. Retrieved 6 December 2016.
- "Kanamycin (Kantrex) Use During Pregnancy". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 7 December 2016.
- Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 302. ISBN 9780471899792.
- World Health Organization (2019). Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva: World Health Organization. hdl:10665/325773. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
- World Health Organization (2019). The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. hdl:10665/330668. ISBN 9789241210300. ISSN 0512-3054. WHO technical report series;1021.
- "Kanamycin (By injection)". Archived from the original on 10 September 2017.
- Briggs G (2011). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. p. 787.
- Consumer Drug Information: Kanamycin, 2 April 2008, archived from the original on 3 May 2008, retrieved 4 May 2008
- "Kanamycin". DrugBank. 17 August 2016.
- "Kanamycin". PubChem. U.S. National Library of Medicine.
- "kanamycin biosynthesis pathway". MetaCyc. Retrieved 30 September 2014.
- "Horizontal Gene Transfer: Plant vs. Bacterial Genes for Antibiotic Resistance Scenario's—What's the Difference?". Isb.vt.edu. Archived from the original on 6 June 2013. Retrieved 24 June 2013.
- Payne NJ, Waghwani HK, Connor MG, Hamilton W, Tockstein S, Moolani H, Chavda F, Badwaik VD, Lawrenz MB, Dakshinamurthy R (May 2016). "Novel Synthesis of Kanamycin Conjugated Gold Nanoparticles with Potent Antibacterial Activity". Frontiers in Microbiology. 7. 607. doi:10.3389/fmicb.2016.00607. PMC 4908860. PMID 27330535.
- Wach A, Brachat A, Pöhlmann R, Philippsen P (December 1994). "New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae". Yeast. 10 (13): 1793–808. doi:10.1002/yea.320101310. PMID 7747518. S2CID 25158247.
- Steiner S, Philippsen P (February 1994). "Sequence and promoter analysis of the highly expressed TEF gene of the filamentous fungus Ashbya gossypii". Molecular & General Genetics. 242 (3): 263–71. doi:10.1007/BF00280415. PMID 8107673. S2CID 19928246.
- Wach A (March 1996). "PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae". Yeast. 12 (3): 259–65. doi:10.1002/(SICI)1097-0061(19960315)12:3<259::AID-YEA901>3.0.CO;2-C. PMID 8904338. S2CID 10450123.
External links
- "Kanamycin A". Drug Information Portal. U.S. National Library of Medicine.