Laminin, alpha 4
Laminin subunit alpha-4 is a protein that in humans is encoded by the LAMA4 gene.[5][6]
| LAMA4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | LAMA4, CMD1JJ, LAMA3, LAMA4*-1, Laminin, alpha 4, laminin subunit alpha 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 600133 MGI: 109321 HomoloGene: 37604 GeneCards: LAMA4 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Function
Laminins, a family of extracellular matrix glycoproteins, are the major noncollagenous constituent of basement membranes. They have been implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signaling, neurite outgrowth and metastasis.
Laminins are composed of 3 non identical chains: laminin alpha, beta and gamma (formerly A, B1, and B2, respectively) and they form a cruciform structure consisting of 3 short arms, each formed by a different chain, and a long arm composed of all 3 chains. Each laminin chain is a multidomain protein encoded by a distinct gene. Several isoforms of each chain have been described. Different alpha, beta and gamma chain isomers combine to give rise to different heterotrimeric laminin isoforms which are designated by Arabic numerals in the order of their discovery, i.e. alpha1beta1gamma1 heterotrimer is laminin 1. The biological functions of the different chains and trimer molecules are largely unknown, but some of the chains have been shown to differ with respect to their tissue distribution, presumably reflecting diverse functions in vivo.
This gene encodes the alpha chain isoform laminin, alpha 4. The domain structure of alpha 4 is similar to that of alpha 3, both of which resemble truncated versions of alpha 1 and alpha 2, in that approximately 1,200 residues at the N-terminus (domains IV, V and VI) have been lost. Laminin, alpha 4 contains the C-terminal G domain which distinguishes all alpha chains from the beta and gamma chains. The RNA analysis from adult and fetal tissues revealed developmental regulation of expression, however, the exact function of laminin, alpha 4 is not known.[6]
Gene
Tissue-specific utilization of alternative polyA-signal has been described in literature. Also, alternative splicing involving the first intron in the 5' UTR, and laminin alpha 4 like isoforms have been noted, however, the full-length nature of these products is not known.[6]
References
- GRCh38: Ensembl release 89: ENSG00000112769 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000019846 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
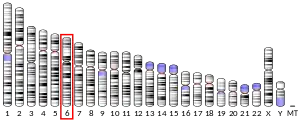
- Richards AJ, al-Imara L, Carter NP, Lloyd JC, Leversha MA, Pope FM (Jul 1994). "Localization of the gene (LAMA4) to chromosome 6q21 and isolation of a partial cDNA encoding a variant laminin A chain". Genomics. 22 (1): 237–9. doi:10.1006/geno.1994.1372. PMID 7959779.
- "Entrez Gene: LAMA4 laminin, alpha 4".
Further reading
- Ljubimova JY, Fujita M, Khazenzon NM, Ljubimov AV, Black KL (2006). "Changes in laminin isoforms associated with brain tumor invasion and angiogenesis". Frontiers in Bioscience. 11 (1): 81–8. doi:10.2741/1781. PMC 3506377. PMID 16146715.
- Iivanainen A, Sainio K, Sariola H, Tryggvason K (May 1995). "Primary structure and expression of a novel human laminin alpha 4 chain". FEBS Letters. 365 (2–3): 183–8. doi:10.1016/0014-5793(95)00462-I. PMID 7781776. S2CID 83904114.
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J (Apr 1994). "A new nomenclature for the laminins". Matrix Biology. 14 (3): 209–11. doi:10.1016/0945-053X(94)90184-8. PMID 7921537.
- Ryan MC, Tizard R, VanDevanter DR, Carter WG (Sep 1994). "Cloning of the LamA3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair". The Journal of Biological Chemistry. 269 (36): 22779–87. doi:10.1016/S0021-9258(17)31713-1. PMID 8077230.
- Richards A, Al-Imara L, Pope FM (Jun 1996). "The complete cDNA sequence of laminin alpha 4 and its relationship to the other human laminin alpha chains". European Journal of Biochemistry. 238 (3): 813–21. doi:10.1111/j.1432-1033.1996.0813w.x. PMID 8706685.
- Xiao S, Lux ML, Reeves R, Hudson TJ, Fletcher JA (Mar 1997). "HMGI(Y) activation by chromosome 6p21 rearrangements in multilineage mesenchymal cells from pulmonary hamartoma". The American Journal of Pathology. 150 (3): 901–10. PMC 1857887. PMID 9060828.
- Durkin ME, Loechel F, Mattei MG, Gilpin BJ, Albrechtsen R, Wewer UM (Jul 1997). "Tissue-specific expression of the human laminin alpha5-chain, and mapping of the gene to human chromosome 20q13.2-13.3 and to distal mouse chromosome 2 near the locus for the ragged (Ra) mutation". FEBS Letters. 411 (2–3): 296–300. doi:10.1016/S0014-5793(97)00686-8. PMID 9271224. S2CID 45286880.
- Richards A, Luccarini C, Pope FM (Aug 1997). "The structural organisation of LAMA4, the gene encoding laminin alpha4". European Journal of Biochemistry. 248 (1): 15–23. doi:10.1111/j.1432-1033.1997.t01-1-00015.x. PMID 9310354.
- Mrowiec T, Melchar C, Górski A (1998). "HIV-protein-mediated alterations in T cell interactions with the extracellular matrix proteins and endothelium". Archivum Immunologiae et Therapiae Experimentalis. 45 (2–3): 255–9. PMID 9597096.
- Talts JF, Sasaki T, Miosge N, Göhring W, Mann K, Mayne R, Timpl R (Nov 2000). "Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues". The Journal of Biological Chemistry. 275 (45): 35192–9. doi:10.1074/jbc.M003261200. PMID 10934193.
- Fleischmajer R, Kuroda K, Utani A, Douglas MacDonald E, Perlish JS, Arikawa-Hirasawa E, Sekiguchi K, Sanzen N, Timpl R, Yamada Y (Dec 2000). "Differential expression of laminin alpha chains during proliferative and differentiation stages in a model for skin morphogenesis". Matrix Biology. 19 (7): 637–47. doi:10.1016/S0945-053X(00)00092-5. PMID 11102753.
- McArthur CP, Wang Y, Heruth D, Gustafson S (Jun 2001). "Amplification of extracellular matrix and oncogenes in tat-transfected human salivary gland cell lines with expression of laminin, fibronectin, collagens I, III, IV, c-myc and p53". Archives of Oral Biology. 46 (6): 545–55. doi:10.1016/S0003-9969(01)00014-0. PMID 11311202.
- Petäjäniemi N, Korhonen M, Kortesmaa J, Tryggvason K, Sekiguchi K, Fujiwara H, Sorokin L, Thornell LE, Wondimu Z, Assefa D, Patarroyo M, Virtanen I (Aug 2002). "Localization of laminin alpha4-chain in developing and adult human tissues". The Journal of Histochemistry and Cytochemistry. 50 (8): 1113–30. doi:10.1177/002215540205000813. PMID 12133914.
- Gu YC, Kortesmaa J, Tryggvason K, Persson J, Ekblom P, Jacobsen SE, Ekblom M (Feb 2003). "Laminin isoform-specific promotion of adhesion and migration of human bone marrow progenitor cells". Blood. 101 (3): 877–85. doi:10.1182/blood-2002-03-0796. PMID 12393739.
- Hayashi Y, Kim KH, Fujiwara H, Shimono C, Yamashita M, Sanzen N, Futaki S, Sekiguchi K (Dec 2002). "Identification and recombinant production of human laminin alpha4 subunit splice variants". Biochemical and Biophysical Research Communications. 299 (3): 498–504. doi:10.1016/S0006-291X(02)02642-6. PMID 12445830.
- Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, Jones JC (Dec 2002). "Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo". Proceedings of the National Academy of Sciences of the United States of America. 99 (25): 16075–80. doi:10.1073/pnas.252649399. PMC 138567. PMID 12454288.
- DeHahn KC, Gonzales M, Gonzalez AM, Hopkinson SB, Chandel NS, Brunelle JK, Jones JC (Mar 2004). "The alpha4 laminin subunit regulates endothelial cell survival". Experimental Cell Research. 294 (1): 281–9. doi:10.1016/j.yexcr.2003.11.006. PMID 14980521.