LARP1
La-related protein 1 (LARP1) is a 150 kDa protein that in humans is encoded by the LARP1 gene.[5][6][7] LARP1 is a novel target of the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway, a circuitry often hyperactivated in cancer which regulates cell growth and proliferation primarily through the regulation of protein synthesis.[8]
Function
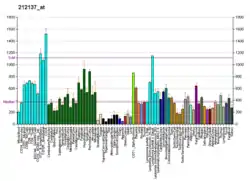
LARP1 is the largest of a 7-member family of LARP proteins (others are: LARP1B, LARP3 (aka genuine La or SSB), LARP4A, LARP4B, LARP6 and LARP7).[9] All LARP proteins, including human LARPs, contain 2 conserved regions. The first conserved region shares homology with La proteins (called the La motif, see SSB) whereas the second conserved region (called the LA- motif) is restricted to LARP proteins. LARP1 and 1B also contain a conserved "DM15 region" within their C-terminus.[10] This region is unique and has been shown to be required for RNA-binding. Mouse Larp1 is expressed in dorsal root ganglia and spinal cord, as well as in developing organs characterized by epithelial-mesenchymal interactions.[6] Human LARP1 is present at low levels in normal, non-embryonic cells but is highly expressed in epithelial cancers (such as ovarian, colorectal, prostate, non-small cell lung, hepatocellular and cervical cancers).[11][12][13][14] Some studies have shown that high levels of LARP1 protein correlate with worse prognosis in cancer patients.[15][16]
LARP1 binds to and regulates the translation of terminal oligopyrimidine motif (TOP mRNAs) and can directly interact with the 5' cap of mRNAs.[17][18] It has also been shown to interact with the 3' end and coding regions (CDS) of other genes.[17] LARP1 protein colocalizes with stress granules and P-bodies,[19] which function in RNA storage and degradation. It has been suggested that LARP1 functions in P-bodies to attenuate the abundance of conserved Ras-MAPK mRNAs. The cluster of LARP1 homologs may function to control the expression of key developmental regulators.[19]
Several studies have demonstrated that LARP1 deficiency selectively affects the recruitment of TOP mRNAs to polysomes. In some cancer cells, LARP1 deficiency reduces proliferation and activates apoptotic cell death.[13] Even though a decrease abundance of proteins encoded by TOP mRNAs has been reported in LARP1 silenced cells, some researchers believe that this can be explained simply by the reduced number of TOP mRNA transcripts in LARP1-deficient cells.
References
- GRCh38: Ensembl release 89: ENSG00000155506 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000037331 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Nagase T, Ishikawa K, Suyama M, Kikuno R, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O (October 1998). "Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 5 (5): 277–86. doi:10.1093/dnares/5.5.277. PMID 9872452.
- Chauvet S, Maurel-Zaffran C, Miassod R, Jullien N, Pradel J, Aragnol D (July 2000). "dlarp, a new candidate Hox target in Drosophila whose orthologue in mouse is expressed at sites of epithelium/mesenchymal interactions". Developmental Dynamics. 218 (3): 401–13. doi:10.1002/1097-0177(200007)218:3<401::AID-DVDY1009>3.0.CO;2-6. PMID 10878606.
- "Entrez Gene: LARP1 La ribonucleoprotein domain family, member 1".
- Tcherkezian J, Cargnello M, Romeo Y, Huttlin EL, Lavoie G, Gygi SP, Roux PP (Feb 2014). "Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5'TOP mRNA translation". Genes Dev. 28 (4): 357–71. doi:10.1101/gad.231407.113. PMC 3937514. PMID 24532714.
- Bousquet-Antonelli C, Deragon JM (May 2009). "A comprehensive analysis of the La-motif protein superfamily". RNA. 15 (5): 750–64. doi:10.1261/rna.1478709. PMC 2673062. PMID 19299548.
- Lahr RM, Mack SM, Héroux A, Blagden SP, Bousquet-Antonelli C, Deragon JM, Berman AJ (September 2015). "The La-related protein 1-specific domain repurposes HEAT-like repeats to directly bind a 5'TOP sequence". Nucleic Acids Research. 43 (16): 8077–88. doi:10.1093/nar/gkv748. PMC 4652764. PMID 26206669.
- Stavraka C, Blagden S (October 2015). "The La-Related Proteins, a Family with Connections to Cancer". Biomolecules. 5 (4): 2701–22. doi:10.3390/biom5042701. PMC 4693254. PMID 26501340.
- Mura M, Hopkins TG, Michael T, Abd-Latip N, Weir J, Aboagye E, Mauri F, Jameson C, Sturge J, Gabra H, Bushell M, Willis AE, Curry E, Blagden SP (September 2015). "LARP1 post-transcriptionally regulates mTOR and contributes to cancer progression". Oncogene. 34 (39): 5025–36. doi:10.1038/onc.2014.428. PMC 4430325. PMID 25531318.
- Hopkins TG, Mura M, Al-Ashtal HA, Lahr RM, Abd-Latip N, Sweeney K, et al. (February 2016). "The RNA-binding protein LARP1 is a post-transcriptional regulator of survival and tumorigenesis in ovarian cancer". Nucleic Acids Research. 44 (3): 1227–46. doi:10.1093/nar/gkv1515. PMC 4756840. PMID 26717985.
- Xie C, Huang L, Xie S, Xie D, Zhang G, Wang P, Peng L, Gao Z (October 2013). "LARP1 predict the prognosis for early-stage and AFP-normal hepatocellular carcinoma". Journal of Translational Medicine. 11: 272. doi:10.1186/1479-5876-11-272. PMC 3814951. PMID 24159927.
- Ye L, Lin ST, Mi YS, Liu Y, Ma Y, Sun HM, Peng ZH, Fan JW (November 2016). "Overexpression of LARP1 predicts poor prognosis of colorectal cancer and is expected to be a potential therapeutic target". Tumour Biology. 37 (11): 14585–14594. doi:10.1007/s13277-016-5332-3. PMC 5126195. PMID 27614686.
- Xu Z, Xu J, Lu H, Lin B, Cai S, Guo J, Zang F, Chen R (December 2017). "LARP1 is regulated by the XIST/miR-374a axis and functions as an oncogene in non-small cell lung carcinoma". Oncology Reports. 38 (6): 3659–3667. doi:10.3892/or.2017.6040. PMID 29039571.
- Hong S, Freeberg MA, Han T, Kamath A, Yao Y, Fukuda T, Suzuki T, Kim JK, Inoki K (June 2017). "LARP1 functions as a molecular switch for mTORC1-mediated translation of an essential class of mRNAs". eLife. 6. doi:10.7554/elife.25237. PMC 5484620. PMID 28650797.
- Lahr RM, Fonseca BD, Ciotti GE, Al-Ashtal HA, Jia JJ, Niklaus MR, Blagden SP, Alain T, Berman AJ (April 2017). "La-related protein 1 (LARP1) binds the mRNA cap, blocking eIF4F assembly on TOP mRNAs". eLife. 6. doi:10.7554/elife.24146. PMC 5419741. PMID 28379136.
- Nykamp K, Lee MH, Kimble J (July 2008). "C. elegans La-related protein, LARP-1, localizes to germline P bodies and attenuates Ras-MAPK signaling during oogenesis". RNA. 14 (7): 1378–89. doi:10.1261/rna.1066008. PMC 2441978. PMID 18515547.
Further reading
- Horke S, Reumann K, Schweizer M, Will H, Heise T (June 2004). "Nuclear trafficking of La protein depends on a newly identified nucleolar localization signal and the ability to bind RNA". The Journal of Biological Chemistry. 279 (25): 26563–70. doi:10.1074/jbc.M401017200. PMID 15060081.
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP (August 2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proceedings of the National Academy of Sciences of the United States of America. 101 (33): 12130–5. Bibcode:2004PNAS..10112130B. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O'Donnell P, Taylor P, Taylor L, Zougman A, Woodgett JR, Langeberg LK, Scott JD, Pawson T (August 2004). "Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization". Current Biology. 14 (16): 1436–50. doi:10.1016/j.cub.2004.07.051. PMID 15324660. S2CID 2371325.
- Beausoleil SA, Villén J, Gerber SA, Rush J, Gygi SP (October 2006). "A probability-based approach for high-throughput protein phosphorylation analysis and site localization". Nature Biotechnology. 24 (10): 1285–92. doi:10.1038/nbt1240. PMID 16964243. S2CID 14294292.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983. S2CID 7827573.