Cathelicidin
Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the CAMP gene encodes the peptide precursor CAP-18 (18 kDa), which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37.[5][6] LL-37 is the only peptide in the Cathelicidin family found in the human body.[7]
| CHAMP1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | CHAMP1, C13orf8, CAMP, CHAMP, ZNF828, MRD40, chromosome alignment maintaining phosphoprotein 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 616327 MGI: 1196398 HomoloGene: 18780 GeneCards: CHAMP1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Cathelicidin peptides are dual-natured molecules called amphiphiles: one end of the molecule is attracted to water and repelled by fats and proteins, and the other end is attracted to fat and proteins and repelled by water. Members of this family react to pathogens by disintegrating, damaging, or puncturing cell membranes.
Cathelicidins thus serve a critical role in mammalian innate immune defense against invasive bacterial infection.[8] The cathelicidin family of peptides are classified as antimicrobial peptides (AMPs). The AMP family also includes the defensins. Whilst the defensins share common structural features, cathelicidin-related peptides are highly heterogeneous.[8] Members of the cathelicidin family of antimicrobial polypeptides are characterized by a highly conserved region (cathelin domain) and a highly variable cathelicidin peptide domain.[8]
Cathelicidin peptides have been isolated from many different species of mammals. Cathelicidins are mostly found in neutrophils, monocytes, mast cells, dendritic cells and macrophages[9] after activation by bacteria, viruses, fungi, parasites or the hormone 1,25-D, which is the hormonally active form of vitamin D.[10] They have been found in some other cells, including epithelial cells and human keratinocytes.[11]
Etymology
The term was coined in 1995 from cathelin, due to the characteristic cathelin-like domain present in cathelicidins.[12] The name cathelin itself is coined from cathepsin L inhibitor in 1989.[13]
Mechanism of antimicrobial activity
The general rule of the mechanism triggering cathelicidin action, like that of other antimicrobial peptides, involves the disintegration (damaging and puncturing) of cell membranes of organisms toward which the peptide is active.[14] Antimicrobial effects have been observed against fungal, bacterial, and viral pathogens.
Cathelicidins rapidly destroy the lipoprotein membranes of microbes enveloped in phagosomes after fusion with lysosomes in macrophages. Therefore, LL-37 can inhibit the formation of bacterial biofilms.[15]
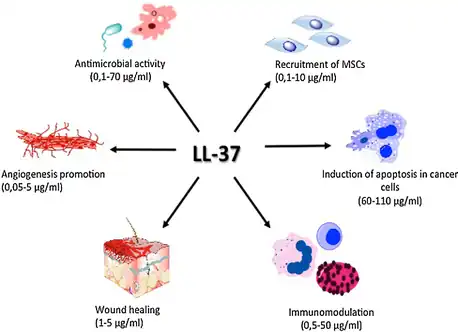
Other activities
LL-37 plays a role in the activation of cell proliferation and migration, contributing to the wound closure process.[16] All these mechanisms together play an essential role in tissue homeostasis and regenerative processes. Moreover, it has an agonistic effect on various pleiotropic receptors, for example, formyl peptide receptor like-1 (FPRL-1),[17] purinergic receptor P2X7, epidermal growth factor receptor (EGFR)[18] or insulin-like growth factor-1 receptor (IGF-1R).[19] These receptors play an important immunomodulatory role in, among other things, anti-tumor immune response.
Furthermore, it induces angiogenesis[20] and regulates apoptosis.[21] These processes are dysregulated during tumor development, and thus LL-37 might be involved in pathogenesis of malignant tumors.
Characteristics
Cathelicidins range in size from 12 to 80 amino acid residues and have a wide range of structures.[22] Most cathelicidins are linear peptides with 23-37 amino acid residues, and fold into amphipathic α-helices. Additionally cathelicidins may also be small-sized molecules (12-18 residues) with beta-hairpin structures, stabilized by one or two disulphide bonds. Even larger cathelicidin peptides (39-80 amino acid residues) are also present. These larger cathelicidins display repetitive proline motifs forming extended polyproline-type structures.[8]
In 1995, Gudmundsson et al. assumed that the active antimicrobial peptide is formed of a 39-residue C-terminal domain (termed FALL-39). However, only a year later stated that the matured AMP, now called LL-37, is in reality two amino acids shorter than FALL-39.[23][24]
The cathelicidin family shares primary sequence homology with the cystatin[25] family of cysteine proteinase inhibitors, although amino acid residues thought to be important in such protease inhibition are usually lacking.
Non-human orthologs
Cathelicidin peptides have been found in humans, monkeys, mice, rats, rabbits, guinea pigs, pandas, pigs, cattle, frogs, sheep, goats, chickens, and horses. Antibodies to the human LL-37/hCAP-18 have been used to find cathelicidin-like compounds in a marsupial.[26] About 30 cathelicidin family members have been described in mammals.
Currently identified cathelicidin peptides include the following:[8]
- Human: hCAP-18 (cleaved into LL-37)
- Rhesus monkey: RL-37
- Mice:CRAMP-1/2, (Cathelicidin-related Antimicrobial Peptide[27]
- Rats: rCRAMP
- Rabbits: CAP-18
- Guinea pig: CAP-11
- Pigs: PR-39, Prophenin, PMAP-23,36,37
- Cattle: BMAP-27,28,34 (Bovine Myeloid Antimicrobial Peptides); Bac5, Bac7
- Frogs: cathelicidin-AL (found in Amolops loloensis)[28]
- Chickens: Four cathelicidins, fowlicidins 1,2,3 and cathelicidin Beta-1 [29]
- Tasmanian Devil: Saha-CATH5 [30]
- Salmonids: CATH1 and CATH2
Clinical significance
Patients with rosacea have elevated levels of cathelicidin and elevated levels of stratum corneum tryptic enzymes (SCTEs). Cathelicidin is cleaved into the antimicrobial peptide LL-37 by both kallikrein 5 and kallikrein 7 serine proteases. Excessive production of LL-37 is suspected to be a contributing cause in all subtypes of Rosacea.[31] Antibiotics have been used in the past to treat rosacea, but antibiotics may only work because they inhibit some SCTEs.[32]
Lower plasma levels of human cathelicidin antimicrobial protein (hCAP18) appear to significantly increase the risk of death from infection in dialysis patients.[33] The production of cathelicidin is up-regulated by vitamin D.[34][35]
SAAP-148 (a synthetic antimicrobial and antibiofilm peptide) is a modified version of LL-37 that has enhanced antimicrobial activities compared to LL-37. In particular, SAAP-148 was more efficient in killing bacteria under physiological conditions.[36] In addition, SAAP-148 synergises with the repurposed antibiotic halicin against antibiotic-resistant bacteria and biofilms.[37]
LL-37 is thought to play a role in psoriasis pathogenesis (along with other anti-microbial peptides). In psoriasis, damaged keratinocytes release LL-37 which forms complexes with self-genetic material (DNA or RNA) from other cells. These complexes stimulate dendritic cells (a type of antigen presenting cell) which then release interferon α and β which contributes to differentiation of T-cells and continued inflammation.[38] LL-37 has also been found to be a common auto-antigen in psoriasis; T-cells specific to LL-37 were found in the blood and skin in two thirds of patients with moderate to severe psoriasis.[38]
LL-37 binds to the peptide Ab, which is associated with Alzheimer's disease. An imbalance between LL-37 and Ab may be a factor affecting AD-associated fibrils and plaques. Chronic, oral Porphyromonas gingivalis, and herpesvirus (HSV-1) infections may contribute to the progression of Alzheimer's dementia.[39][40]
Applications
Research into the AMP family—particularly in regards to their mechanism of action—has been ongoing for nearly 20 years. Despite sustained interest, treatments derived or utilizing AMPs have not been widely adopted for clinical use for several reasons.[41] One, drug candidates from AMPs have a narrow window of bioavailability, because peptides are quickly broken down by proteases. Two, peptide drugs are more expensive than small molecule drugs to produce, which is problematic since peptide drugs must be given in large doses to counter rapid enzymatic breakdown. These qualities also limit routes of administration, typically to injection, infusion, or slow release therapy.[42]
References
- GRCh38: Ensembl release 89: ENSG00000198824 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000047710 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: CAMP cathelicidin antimicrobial peptide".
- UniProt entry: P49913 Retrieved 29 November 2019
- Dürr U, Sudheendra U, Ramamoorthy, A (September 2006). "LL-37, the only human member of the cathelicidin family of antimicrobial peptides". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1758 (9): 1408–1425. doi:10.1016/j.bbamem.2006.03.030. PMID 16716248.
- Zanetti M (January 2004). "Cathelicidins, multifunctional peptides of the innate immunity". Journal of Leukocyte Biology. 75 (1): 39–48. doi:10.1189/jlb.0403147. PMID 12960280. S2CID 14902156.
- Vandamme D, Landuyt B, Luyten W, Schoofs L (November 2012). "A comprehensive summary of LL-37, the factotum human cathelicidin peptide". Cellular Immunology. 280 (1): 22–35. doi:10.1016/j.cellimm.2012.11.009. PMID 23246832.
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. (March 2006). "Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response". Science. 311 (5768): 1770–3. Bibcode:2006Sci...311.1770L. doi:10.1126/science.1123933. PMID 16497887. S2CID 52869005.
- Bals R, Wang X, Zasloff M, Wilson JM (August 1998). "The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9541–6. Bibcode:1998PNAS...95.9541B. doi:10.1073/pnas.95.16.9541. PMC 21374. PMID 9689116.
- Zanetti M, Gennaro R, Romeo D (October 1995). "Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain". FEBS Letters. 374 (1): 1–5. doi:10.1016/0014-5793(95)01050-o. PMID 7589491. S2CID 34865828.
- Ritonja A, Kopitar M, Jerala R, Turk V (September 1989). "Primary structure of a new cysteine proteinase inhibitor from pig leucocytes". FEBS Letters. 255 (2): 211–4. doi:10.1016/0014-5793(89)81093-2. PMID 2792375.
- Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, et al. (December 2012). "Cathelicidins: family of antimicrobial peptides. A review". Molecular Biology Reports. 39 (12): 10957–70. doi:10.1007/s11033-012-1997-x. PMC 3487008. PMID 23065264.
- Dosler S, Karaaslan E (December 2014). "Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides". Peptides. 62: 32–7. doi:10.1016/j.peptides.2014.09.021. PMID 25285879. S2CID 207359996.
- Shaykhiev R, Beisswenger C, Kändler K, Senske J, Püchner A, Damm T, et al. (November 2005). "Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure". American Journal of Physiology. Lung Cellular and Molecular Physiology. 289 (5): L842-8. doi:10.1152/ajplung.00286.2004. PMID 15964896.
- Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O (October 2000). "LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells". The Journal of Experimental Medicine. 192 (7): 1069–74. doi:10.1084/jem.192.7.1069. PMC 2193321. PMID 11015447.
- von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, et al. (January 2008). "The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells". Lung Cancer. 59 (1): 12–23. doi:10.1016/j.lungcan.2007.07.014. PMID 17764778.
- Girnita A, Zheng H, Grönberg A, Girnita L, Ståhle M (January 2012). "Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor". Oncogene. 31 (3): 352–65. doi:10.1038/onc.2011.239. PMC 3262900. PMID 21685939.
- Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, et al. (June 2003). "An angiogenic role for the human peptide antibiotic LL-37/hCAP-18". The Journal of Clinical Investigation. 111 (11): 1665–72. doi:10.1172/JCI17545. PMC 156109. PMID 12782669.
- Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, et al. (2013-05-20). Nie D (ed.). "FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells". PLOS ONE. 8 (5): e63641. Bibcode:2013PLoSO...863641R. doi:10.1371/journal.pone.0063641. PMC 3659029. PMID 23700428.
- Gennaro R, Zanetti M (2000). "Structural features and biological activities of the cathelicidin-derived antimicrobial peptides". Biopolymers. 55 (1): 31–49. doi:10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. PMID 10931440.
- Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH (January 1995). "FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis". Proceedings of the National Academy of Sciences of the United States of America. 92 (1): 195–9. Bibcode:1995PNAS...92..195A. doi:10.1073/pnas.92.1.195. PMC 42844. PMID 7529412.
- Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R (June 1996). "The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes". European Journal of Biochemistry. 238 (2): 325–32. doi:10.1111/j.1432-1033.1996.0325z.x. PMID 8681941.
- Zaiou M, Nizet V, Gallo RL (May 2003). "Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence". The Journal of Investigative Dermatology. 120 (5): 810–6. doi:10.1046/j.1523-1747.2003.12132.x. PMID 12713586.
- Carman RL, Simonian MR, Old JM, Jacques NA, Deane EM (December 2008). "Immunohistochemistry using antibodies to the cathelicidin LL37/hCAP18 in the tammar wallaby, Macropus eugenii". Tissue & Cell. 40 (6): 459–466. doi:10.1016/j.tice.2008.05.002. PMID 18597803.
- Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R (May 1997). "Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse". The Journal of Biological Chemistry. 272 (20): 13088–93. doi:10.1074/jbc.272.20.13088. PMID 9148921.
- Hao X, Yang H, Wei L, Yang S, Zhu W, Ma D, Yu H, Lai R (August 2012). "Amphibian cathelicidin fills the evolutionary gap of cathelicidin in vertebrate". Amino Acids. 43 (2): 677–85. doi:10.1007/s00726-011-1116-7. PMID 22009138. S2CID 2794908.
- Achanta M, Sunkara LT, Dai G, Bommineni YR, Jiang W, Zhang G (May 2012). "Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides". Journal of Animal Science and Biotechnology. 3 (1): 15. doi:10.1186/2049-1891-3-15. PMC 3436658. PMID 22958518.
- Peel E, Cheng Y, Djordjevic JT, Fox S, Sorrell TC, Belov K (October 2016). "Cathelicidins in the Tasmanian devil (Sarcophilus harrisii)". Scientific Reports. 6: 35019. Bibcode:2016NatSR...635019P. doi:10.1038/srep35019. PMC 5057115. PMID 27725697.
- Reinholz M, Ruzicka T, Schauber J (May 2012). "Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease". Annals of Dermatology. 24 (2): 126–35. doi:10.5021/ad.2012.24.2.126. PMC 3346901. PMID 22577261.
- Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL (August 2007). "Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea". Nature Medicine. 13 (8): 975–80. doi:10.1038/nm1616. PMID 17676051. S2CID 23470611.
- Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Koeffler HP, Thadhani R (February 2009). "Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis". Clinical Infectious Diseases. 48 (4): 418–24. doi:10.1086/596314. PMC 6944311. PMID 19133797.
- Zasloff M (January 2002). "Antimicrobial peptides of multicellular organisms". Nature. 415 (6870): 389–95. Bibcode:2002Natur.415..389Z. doi:10.1038/415389a. PMID 11807545. S2CID 205028607.
- Kamen DL, Tangpricha V (May 2010). "Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity". Journal of Molecular Medicine. 88 (5): 441–50. doi:10.1007/s00109-010-0590-9. PMC 2861286. PMID 20119827.
- de Breij A, Riool M, Cordfunke RA, Malanovic N, de Boer L, Koning RI, et al. (January 2018). "The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms". Science Translational Medicine. 10 (423): eaan4044. doi:10.1126/scitranslmed.aan4044. PMID 29321257.
- van Gent ME, van der Reijden TJ, Lennard PR, de Visser AW, Schonkeren-Ravensbergen B, Dolezal N, et al. (May 2022). "Synergism between the Synthetic Antibacterial and Antibiofilm Peptide (SAAP)-148 and Halicin". Antibiotics. 11 (5): 673. doi:10.3390/ANTIBIOTICS11050673.
- Rendon A, Schäkel K (March 2019). "Psoriasis Pathogenesis and Treatment". International Journal of Molecular Sciences. 20 (6): 1475. doi:10.3390/ijms20061475. PMC 6471628. PMID 30909615.
- Kanagasingam S, Chukkapalli SS, Welbury R, Singhrao SK (December 2020). "Porphyromonas gingivalis is a Strong Risk Factor for Alzheimer's Disease". Journal of Alzheimer's Disease Reports. 4 (1): 501–511. doi:10.3233/ADR-200250. PMC 7835991. PMID 33532698.
- Rizzo R (June 2020). Spindler KR (ed.). "Controversial role of herpesviruses in Alzheimer's disease". PLOS Pathogens. 16 (6): e1008575. doi:10.1371/journal.ppat.1008575. PMC 7302436. PMID 32555685.
- "Biomimetics Research from the Barron Lab". web.stanford.edu. Retrieved 2021-10-22.
- DePalma A (2015-06-30). "Peptides: New Processes, Lower Costs". GEN - Genetic Engineering and Biotechnology News. Retrieved 2021-11-30.
Further reading
- Dürr UH, Sudheendra US, Ramamoorthy A (September 2006). "LL-37, the only human member of the cathelicidin family of antimicrobial peptides". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1758 (9): 1408–25. doi:10.1016/j.bbamem.2006.03.030. PMID 16716248.
- Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, Hökfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A (June 2006). "The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection". Nature Medicine. 12 (6): 636–41. doi:10.1038/nm1407. PMID 16751768. S2CID 20704807.
- Gombart AF, Borregaard N, Koeffler HP (July 2005). "Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3". FASEB Journal. 19 (9): 1067–77. doi:10.1096/fj.04-3284com. PMID 15985530. S2CID 7563259.
- López-García B, Lee PH, Gallo RL (May 2006). "Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor". The Journal of Antimicrobial Chemotherapy. 57 (5): 877–82. doi:10.1093/jac/dkl078. PMID 16556635.
- Lehrer RI, Ganz T (January 2002). "Cathelicidins: a family of endogenous antimicrobial peptides". Current Opinion in Hematology. 9 (1): 18–22. doi:10.1097/00062752-200201000-00004. PMID 11753073. S2CID 23575052.
- Niyonsaba F, Hirata M, Ogawa H, Nagaoka I (September 2003). "Epithelial cell-derived antibacterial peptides human beta-defensins and cathelicidin: multifunctional activities on mast cells". Current Drug Targets. Inflammation and Allergy. 2 (3): 224–31. doi:10.2174/1568010033484115. PMID 14561157.
- van Wetering S, Tjabringa GS, Hiemstra PS (April 2005). "Interactions between neutrophil-derived antimicrobial peptides and airway epithelial cells". Journal of Leukocyte Biology. 77 (4): 444–50. doi:10.1189/jlb.0604367. PMID 15591123. S2CID 8261526.
- Cowland JB, Johnsen AH, Borregaard N (July 1995). "hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules". FEBS Letters. 368 (1): 173–6. doi:10.1016/0014-5793(95)00634-L. PMID 7615076. S2CID 3172761.
- Gudmundsson GH, Magnusson KP, Chowdhary BP, Johansson M, Andersson L, Boman HG (July 1995). "Structure of the gene for porcine peptide antibiotic PR-39, a cathelin gene family member: comparative mapping of the locus for the human peptide antibiotic FALL-39". Proceedings of the National Academy of Sciences of the United States of America. 92 (15): 7085–9. Bibcode:1995PNAS...92.7085G. doi:10.1073/pnas.92.15.7085. PMC 41476. PMID 7624374.
- Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC (April 1995). "Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein". Infection and Immunity. 63 (4): 1291–7. doi:10.1128/IAI.63.4.1291-1297.1995. PMC 173149. PMID 7890387.
- Larrick JW, Lee J, Ma S, Li X, Francke U, Wright SC, Balint RF (November 1996). "Structural, functional analysis and localization of the human CAP18 gene". FEBS Letters. 398 (1): 74–80. doi:10.1016/S0014-5793(96)01199-4. PMID 8946956. S2CID 35329283.
- Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, Gudmundsson GH (June 1997). "The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders". The Journal of Biological Chemistry. 272 (24): 15258–63. doi:10.1074/jbc.272.24.15258. PMID 9182550.
- Bals R, Wang X, Zasloff M, Wilson JM (August 1998). "The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9541–6. Bibcode:1998PNAS...95.9541B. doi:10.1073/pnas.95.16.9541. PMC 21374. PMID 9689116.
- Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O (October 2000). "LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells". The Journal of Experimental Medicine. 192 (7): 1069–74. doi:10.1084/jem.192.7.1069. PMC 2193321. PMID 11015447.
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jörnvall H, Wigzell H, Gudmundsson GH (November 2000). "The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations". Blood. 96 (9): 3086–93. doi:10.1182/blood.V96.9.3086. PMID 11049988.
- Bals R, Lang C, Weiner DJ, Vogelmeier C, Welsch U, Wilson JM (March 2001). "Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules". Clinical and Diagnostic Laboratory Immunology. 8 (2): 370–5. doi:10.1128/CDLI.8.2.370-375.2001. PMC 96065. PMID 11238224.
- Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D (September 2001). "Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells". Journal of Immunology. 167 (6): 3329–38. doi:10.4049/jimmunol.167.6.3329. PMID 11544322.
- Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF (February 2002). "Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium". Infection and Immunity. 70 (2): 953–63. doi:10.1128/IAI.70.2.953-963.2002. PMC 127717. PMID 11796631.
- Giuliani A, Pirri G, Nicoletto S (2007). "Antimicrobial peptides: an overview of a promising class of therapeutics". Cent. Eur. J. Biol. 2 (1): 1–33. doi:10.2478/s11535-007-0010-5.
- Burton MF, Steel PG (December 2009). "The chemistry and biology of LL-37". Natural Product Reports. 26 (12): 1572–84. doi:10.1039/b912533g. PMID 19936387.