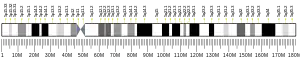Lysyl oxidase
Lysyl oxidase (LOX), also known as protein-lysine 6-oxidase, is an enzyme that, in humans, is encoded by the LOX gene.[5][6] It catalyzes the conversion of lysine residues into its aldehyde derivative allysine.[7] Allysine form cross-links in extracellular matrix proteins. Inhibition of lysyl oxidase can cause osteolathyrism, but, at the same time, its upregulation by tumor cells may promote metastasis of the existing tumor, causing it to become malignant and cancerous.
Structure
In the yeast species Pichia pastoris, lysyl oxidase constitutes a homodimeric structure. Each monomer consists of an active site that includes a Cu(II) atom, coordinated by three histidine residues, as well as 2,4,5-trihydroxyphenylalanine quinone (TPQ), a crucial cofactor.[8]
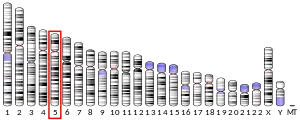
In humans, the LOX gene is located on chromosome 5 q23.3-31.2. The DNA sequence encodes a polypeptide of 417 amino acids, the first 21 residues of which constitute a signal peptide,[6] with a weight of approximately 32 kDa.[9] The carboxyterminus contains the active copper (II) ion, lysine, tyrosine, and cysteine residues that comprise the catalytically active site.[10] The three-dimensional structure of human lysyl oxidase has not yet been resolved.[11]
Mechanism

Lysyl oxidase the terminal carbon of the side chain of lysyl residue side chain.[9] The enzyme belongsthe category of quinone-containing copper amine oxidases. The reaction requires the cofactor lysyl tyrosylquinone (LTQ). The LTQ cofactor is unique among quinones because it contains an 1,2-benzoquinone substituent. Furthermore, it is neutral charge at physiological pH.[12][13] The ε-amine is condenses with LTQ to give the Schiff base via reaction with LTQ. The rate-limiting removal of a ε-proton yields an imine. Subsequent hydrolysis of the imine leads to release of the allysine residue. Molecular oxygen and the copper ion are utilized to reoxidize the cofactor, producing hydrogen peroxide as a side product.[14]

Biological function
Lysyl oxidase is an extracellular copper-dependent enzyme that catalyzes formation of aldehydes from lysine residues in collagen and elastin precursors.[15][16] These aldehydes react with unmodified lysine residues, resulting in cross-linking collagen and elastin, which is essential for stabilization of collagen fibrils and for the integrity and elasticity of mature elastin.[5]
Complex cross-links are formed in collagen (pyridinolines derived from three lysine residues) and in elastin (desmosines derived from four lysine residues) that differ in structure.[17]
The importance of lysyl oxidase-derived cross-linking was established from animal studies in which lysyl oxidase was inhibited either by nutritional copper-deficiency or by supplementation of diets with β-aminopropionitrile (BAPN), an inhibitor of lysyl oxidase.[18] This resulted in lathyrism, characterized by poor bone formation and strength, hyperextensible skin, weak ligaments, and increased occurrence of aortic aneurysms. These abnormalities correlated well with decreased cross-linking of collagen and elastin.[19]
Developmentally, reduced lysyl oxidase activity have been implicated in Menkes disease and occipital horn syndrome, two X-linked recessive disorders characterized by a mutation in a gene coding for a protein involved in copper transport. Thus, not only is LOX crucial to cardiovascular development, it plays a major role in connective tissue development and may also be important in neurological function.[20]
Lysyl oxidase has also proven crucial to the development of the respiratory system and the skin, as collagen and elastin represent 50-60% of the composition of the lung, and 75% of the skin. In Lox homozygous null models (Lox -/-), the activity of LOX was reduced by up to 80%, and the phenotype of the lungs resembles those of patients with emphysema and dilated distal airways.[20]
Lysyl oxidase plays a crucial role in the commitment step of adipocyte, or fat cell, formation from pluripotent stem cells during development. Its absence may lead to defects in the transforming growth factor beta superfamily of proteins, which control cell growth and differentiation.[21]
Clinical significance
LOX expression is regulated by hypoxia-inducible factors (HIFs), and, hence, LOX expression is often upregulated in hypoxic breast and head and neck tumors. Patients with high LOX-expressing tumors have poor overall survival. Furthermore, inhibition of LOX has been demonstrated to eliminate metastases in mice. Secreted LOX is responsible for the invasive properties of hypoxic cancer cells through focal adhesion kinase activity and cell-to-matrix adhesion. LOX may be required to create a niche permissive for metastatic growth and, thus, may be required for hypoxia-induced metastasis.[22] In fact, recent research has shown overexpression of LOX as crucial to promoting tumor growth and metastasis in several cancers, including breast cancer,[23] non-small cell lung cancer,[24] and colorectal cancer.[25]
LOX expression was also detected in megakaryocytes, or bone marrow cells responsible for the production of platelets. Data derived from a mouse model of myelofibrosis implicated LOX in bone marrow fibrosis.
In a rodent model of breast cancer, a small-molecule or antibody inhibitors of LOX abolished metastasis.[26] LOX secreted by hypoxic breast tumor cells crosslinks collagen in the basement membrane and is essential for CD11b+ myeloid cell recruitment. CD11b+ cells in turn adhere to crosslinked collagen and produce matrix metalloproteinase-2, which cleaves collagen, enhancing the invasion of metastasizing tumor cells. In contrast, LOX inhibition prevents CD11b+ cell recruitment and metastatic growth.[27]
In cells lacking TGF-β receptors, a deficiency that is characteristic of lung cancer, lysyl oxidase is found in high concentrations. LOX immunostaining has revealed that high LOX expression is associated with high extent of carcinoma invasion in samples obtained from surgically removed lung adenocarcinomas. Additionally, LOX expression is an indicator of 5-year survival in patients, with a 71% chance of survival for patients with low LOX levels, compared to 43% for patients with high LOX levels. Thus, upregulation of lysyl oxidase is a predictor of poor prognosis in early-stage adenocarcinoma patients.[28]
Lysyl oxidase has been newly implicated in tumor angiogenesis, or blood vessel formation, both in vivo and in vitro. Subcutaneous tumor-derived LOX was shown to increase vascular endothelial growth factor (VEGF) expression and secretion, which then promotes angiogenesis by phosphorylation of protein kinase B, or Akt, through platelet-derived growth factor receptor β (PDGFRB). High levels of LOX were associated with high blood vessel density in patient samples. Clinically relevant LOX inhibitors may help slow cancer progression by downregulating crucial growth factors that promote solid tumor progression.[29]
Hence, inhibitors of the LOX enzyme may be useful in preventing angiogenesis, tumor progression, and metastasis as well as treating other fibrotic disease involving remodeling of the extracellular matrix, including neurodegenerative and cardiovascular diseases.[30]
References
- GRCh38: Ensembl release 89: ENSG00000113083 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024529 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: LOX lysyl oxidase".
- Hämäläinen ER, Jones TA, Sheer D, Taskinen K, Pihlajaniemi T, Kivirikko KI (Nov 1991). "Molecular cloning of human lysyl oxidase and assignment of the gene to chromosome 5q23.3-31.2". Genomics. 11 (3): 508–16. doi:10.1016/0888-7543(91)90057-L. PMID 1685472.
- Requena, J. R.; Levine, R. L.; Stadtman, E. R. (2003). "Recent Advances in the Analysis of Oxidized Proteins". Amino Acids. 25 (3–4): 221–226. doi:10.1007/s00726-003-0012-1. PMID 14661085. S2CID 28837698.
- Duff AP, Cohen AE, Ellis PJ, Kuchar JA, Langley DB, Shepard EM, Dooley DM, Freeman HC, Guss JM (Dec 2003). "The crystal structure of Pichia pastoris lysyl oxidase". Biochemistry. 42 (51): 15148–57. doi:10.1021/bi035338v. PMID 14690425.
- Gacheru SN, Trackman PC, Shah MA, O'Gara CY, Spacciapoli P, Greenaway FT, Kagan HM (Nov 1990). "Structural and catalytic properties of copper in lysyl oxidase". The Journal of Biological Chemistry. 265 (31): 19022–7. doi:10.1016/0162-0134(89)84532-5. PMID 1977746.
- Thomassin L, Werneck CC, Broekelmann TJ, Gleyzal C, Hornstra IK, Mecham RP, Sommer P (Dec 2005). "The Pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers". The Journal of Biological Chemistry. 280 (52): 42848–55. doi:10.1074/jbc.M506832200. PMID 16251195.
- Kagan HM, Li W (Mar 2003). "Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell". Journal of Cellular Biochemistry. 88 (4): 660–72. doi:10.1002/jcb.10413. PMID 12577300. S2CID 23651213.
- Wang SX, Nakamura N, Mure M, Klinman JP, Sanders-Loehr J (Nov 1997). "Characterization of the Native Lysine Tyrosylquinone Cofactor in Lysyl Oxidase by Raman Spectroscopy". The Journal of Biological Chemistry. 272 (46): 28841–4. doi:10.1074/jbc.272.46.28841. PMID 9360949.
- Bedell-Hogan D, Trackman P, Abrams W, Rosenbloom J, Kagan H (May 1993). "Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase". The Journal of Biological Chemistry. 268 (14): 10345–50. doi:10.1016/S0021-9258(18)82207-4. PMID 8098038.
- Akagawa M, Suyama K (Feb 2001). "Characterization of a model compound for the lysine tyrosylquinone cofactor of lysyl oxidase". Biochemical and Biophysical Research Communications. 281 (1): 193–9. doi:10.1006/bbrc.2001.4315. PMID 11178979.
- Alberts, Bruce (2002). Molecular biology of the cell. New York: Garland Science. p. 1099. ISBN 978-0-8153-3218-3.
- Csiszar K (2001). Lysyl oxidases: a novel multifunctional amine oxidase family. Progress in Nucleic Acid Research and Molecular Biology. Vol. 70. pp. 1–32. doi:10.1016/S0079-6603(01)70012-8. ISBN 9780125400701. PMID 11642359.
- Siegel RC, Fu JC, Uto N, Horiuchi K, Fujimoto D (Oct 1982). "Collagen cross-linking: lysyl oxidase dependent synthesis of pyridinoline in vitro: confirmation that pyridinoline is derived from collagen". Biochemical and Biophysical Research Communications. 108 (4): 1546–50. doi:10.1016/S0006-291X(82)80083-1. PMID 6129847.
- Dawson DA, Rinaldi AC, Pöch G (Aug 2002). "Biochemical and toxicological evaluation of agent-cofactor reactivity as a mechanism of action for osteolathyrism". Toxicology. 177 (2–3): 267–84. doi:10.1016/S0300-483X(02)00233-0. PMID 12135629.
- Wilmarth KR, Froines JR (Nov 1992). "In vitro and in vivo inhibition of lysyl oxidase by aminopropionitriles". Journal of Toxicology and Environmental Health. 37 (3): 411–23. doi:10.1080/15287399209531680. PMID 1359158.
- Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J (Oct 2005). "Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues". The American Journal of Pathology. 167 (4): 927–36. doi:10.1016/S0002-9440(10)61183-2. PMC 1603668. PMID 16192629.
- Huang HY, Chen SZ, Zhang WT, Wang SS, Liu Y, Li X, Sun X, Li YM, Wen B, Lei QY, Tang QQ (May 2013). "Induction of epithelial-mesenchymal transition (EMT)-like response by BMP4 via up-regulation of lysyl oxidase is required for adipocyte lineage commitment". Stem Cell Research. 10 (3): 278–287. doi:10.1016/j.scr.2012.12.005. PMID 23395997.
- Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ (Apr 2006). "Lysyl oxidase is essential for hypoxia-induced metastasis". Nature. 440 (7088): 1222–6. doi:10.1038/nature04695. PMID 16642001. S2CID 4429932.
- El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, Csizmadia E, Mariani O, Zhu C, Campagne A, Toner M, Bhatia SN, Irimia D, Vincent-Salomon A, Karnoub AE (Oct 2012). "Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy". Proceedings of the National Academy of Sciences of the United States of America. 109 (43): 17460–5. doi:10.1073/pnas.1206653109. PMC 3491529. PMID 23033492.
- Shi W, Yang B, Li X, Sun S, Wang L, Jiao S (Dec 2012). "The effect of lysyl oxidase polymorphism on susceptibility and prognosis of nonsmall cell lung cancer". Tumour Biology. 33 (6): 2379–83. doi:10.1007/s13277-012-0501-5. PMID 22948781. S2CID 14633716.
- Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun XF, Southall SM, Wilson JR, Erler JT (Mar 2011). "The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer". Journal of the National Cancer Institute. 103 (5): 407–24. doi:10.1093/jnci/djq569. PMID 21282564.
- Erler JT, Giaccia AJ (Nov 2006). "Lysyl oxidase mediates hypoxic control of metastasis". Cancer Research. 66 (21): 10238–41. doi:10.1158/0008-5472.CAN-06-3197. PMID 17079439.
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ (Jan 2009). "Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche". Cancer Cell. 15 (1): 35–44. doi:10.1016/j.ccr.2008.11.012. PMC 3050620. PMID 19111879.
- Wilgus ML, Borczuk AC, Stoopler M, Ginsburg M, Gorenstein L, Sonett JR, Powell CA (May 2011). "Lysyl oxidase: a lung adenocarcinoma biomarker of invasion and survival". Cancer. 117 (10): 2186–91. doi:10.1002/cncr.25768. PMID 21523732. S2CID 25144943.
- Baker AM, Bird D, Welti JC, Gourlaouen M, Lang G, Murray GI, Reynolds AR, Cox TR, Erler JT (Jan 2013). "Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis". Cancer Research. 73 (2): 583–94. doi:10.1158/0008-5472.CAN-12-2447. PMC 3548904. PMID 23188504.
- Rodríguez C, Rodríguez-Sinovas A, Martínez-González J (May 2008). "Lysyl oxidase as a potential therapeutic target". Drug News & Perspectives. 21 (4): 218–24. doi:10.1358/dnp.2008.21.4.1213351. PMID 18560621.
Further reading
- Csiszar K (2001). Lysyl oxidases: a novel multifunctional amine oxidase family. Progress in Nucleic Acid Research and Molecular Biology. Vol. 70. pp. 1–32. doi:10.1016/S0079-6603(01)70012-8. ISBN 9780125400701. PMID 11642359.
- Kagan HM, Li W (Mar 2003). "Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell". Journal of Cellular Biochemistry. 88 (4): 660–72. doi:10.1002/jcb.10413. PMID 12577300. S2CID 23651213.
- Svinarich DM, Twomey TA, Macauley SP, Krebs CJ, Yang TP, Krawetz SA (Jul 1992). "Characterization of the human lysyl oxidase gene locus". The Journal of Biological Chemistry. 267 (20): 14382–7. doi:10.1016/S0021-9258(19)49723-8. PMID 1352776.
- Mariani TJ, Trackman PC, Kagan HM, Eddy RL, Shows TB, Boyd CD, Deak SB (Jun 1992). "The complete derived amino acid sequence of human lysyl oxidase and assignment of the gene to chromosome 5 (extensive sequence homology with the murine ras recision gene)". Matrix. 12 (3): 242–8. doi:10.1016/S0934-8832(11)80067-3. PMID 1357535.
- Murawaki Y, Kusakabe Y, Hirayama C (Dec 1991). "Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin". Hepatology. 14 (6): 1167–73. doi:10.1002/hep.1840140635. PMID 1683640. S2CID 25820738.
- Hämäläinen ER, Jones TA, Sheer D, Taskinen K, Pihlajaniemi T, Kivirikko KI (Nov 1991). "Molecular cloning of human lysyl oxidase and assignment of the gene to chromosome 5q23.3-31.2". Genomics. 11 (3): 508–16. doi:10.1016/0888-7543(91)90057-L. PMID 1685472.
- Konishi A, Iguchi H, Ochi J, Kinoshita R, Miura K, Uchino H (Oct 1985). "Increased lysyl oxidase activity in culture medium of nonparenchymal cells from fibrotic livers". Gastroenterology. 89 (4): 709–15. doi:10.1016/0016-5085(85)90563-3. PMID 2863189.
- Kuivaniemi H, Ala-Kokko L, Kivirikko KI (Sep 1986). "Secretion of lysyl oxidase by cultured human skin fibroblasts and effects of monensin, nigericin, tunicamycin and colchicine". Biochimica et Biophysica Acta (BBA) - General Subjects. 883 (2): 326–34. doi:10.1016/0304-4165(86)90325-9. PMID 2874833.
- Reiser KM, Hennessy SM, Last JA (Dec 1987). "Analysis of age-associated changes in collagen crosslinking in the skin and lung in monkeys and rats". Biochimica et Biophysica Acta (BBA) - General Subjects. 926 (3): 339–48. doi:10.1016/0304-4165(87)90220-0. PMID 3120785.
- Järveläinen H, Halme T, Rönnemaa T (1982). "Effect of cortisol on the proliferation and protein synthesis of human aortic smooth muscle cells in culture". Acta Medica Scandinavica. Supplementum. 660: 114–22. doi:10.1111/j.0954-6820.1982.tb00367.x. PMID 6127904.
- Kuivaniemi H, Savolainen ER, Kivirikko KI (Jun 1984). "Human placental lysyl oxidase. Purification, partial characterization, and preparation of two specific antisera to the enzyme". The Journal of Biological Chemistry. 259 (11): 6996–7002. doi:10.1016/S0021-9258(17)39828-9. PMID 6144680.
- Lien YH, Stern R, Fu JC, Siegel RC (Sep 1984). "Inhibition of collagen fibril formation in vitro and subsequent cross-linking by glucose". Science. 225 (4669): 1489–91. doi:10.1126/science.6147899. PMID 6147899.
- Yasutake A, Powers JC (Jun 1981). "Reactivity of human leukocyte elastase and porcine pancreatic elastase toward peptide 4-nitroanilides containing model desmosine residues. Evidence that human leukocyte elastase is selective for cross-linked regions of elastin". Biochemistry. 20 (13): 3675–9. doi:10.1021/bi00516a002. PMID 6912069.
- Kim Y, Boyd CD, Csiszar K (Mar 1995). "A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase". The Journal of Biological Chemistry. 270 (13): 7176–82. doi:10.1074/jbc.270.13.7176. PMID 7706256.
- Hämäläinen ER, Kemppainen R, Pihlajaniemi T, Kivirikko KI (Sep 1993). "Structure of the human lysyl oxidase gene". Genomics. 17 (3): 544–8. doi:10.1006/geno.1993.1369. PMID 7902322.
- Forbes EG, Cronshaw AD, MacBeath JR, Hulmes DJ (Sep 1994). "Tyrosine-rich acidic matrix protein (TRAMP) is a tyrosine-sulphated and widely distributed protein of the extracellular matrix". FEBS Letters. 351 (3): 433–6. doi:10.1016/0014-5793(94)00907-4. PMID 8082810. S2CID 23360856.
- Csiszar K, Mariani TJ, Gosin JS, Deak SB, Boyd CD (May 1993). "A restriction fragment length polymorphism results in a nonconservative amino acid substitution encoded within the first exon of the human lysyl oxidase gene". Genomics. 16 (2): 401–6. doi:10.1006/geno.1993.1203. PMID 8100215.
- Vetter U, Weis MA, Mörike M, Eanes ED, Eyre DR (Feb 1993). "Collagen crosslinks and mineral crystallinity in bone of patients with osteogenesis imperfecta". Journal of Bone and Mineral Research. 8 (2): 133–7. doi:10.1002/jbmr.5650080203. PMID 8442432. S2CID 21904627.
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM (Mar 1996). "Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase". The Journal of Biological Chemistry. 271 (12): 7113–9. doi:10.1074/jbc.271.12.7113. PMID 8636146.
- Khakoo A, Thomas R, Trompeter R, Duffy P, Price R, Pope FM (Feb 1997). "Congenital cutis laxa and lysyl oxidase deficiency". Clinical Genetics. 51 (2): 109–14. doi:10.1111/j.1399-0004.1997.tb02430.x. PMID 9111998. S2CID 36246895.
- Smithen DA, Leung LM, Challinor M, Lawrence R, Tang H, Niculescu-Duvaz D, et al. (March 2020). "2-Aminomethylene-5-sulfonylthiazole Inhibitors of Lysyl Oxidase (LOX) and LOXL2 Show Significant Efficacy in Delaying Tumor Growth". Journal of Medicinal Chemistry. 63 (5): 2308–2324. doi:10.1021/acs.jmedchem.9b01112. PMC 7073924. PMID 31430136.
External links
- Lysyl+Oxidase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)