Lead(II) chromate
Lead(II) chromate is the inorganic compound with the formula (PbCrO4). It has a vivid yellow color and is generally insoluble. Two polymorphs of lead chromate are known, orthorhombic and the more stable monoclinic form. Monoclinic lead chromate is used in paints under the name chrome yellow, and many other names.[1] It occurs also as the mineral crocoite.
 | |
 | |
| Names | |
|---|---|
| Other names
see text | |
| Identifiers | |
| ChEBI | |
| ECHA InfoCard | 100.028.951 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3288 |
CompTox Dashboard (EPA) |
|
| Properties | |
| PbCrO4 | |
| Molar mass | 323.192 g/mol |
| Appearance | orange-yellow powder |
| Density | 6.12 g/cm3, solid |
| Melting point | 844 °C (1,551 °F; 1,117 K) |
| negligible | |
| Solubility | soluble in diluted nitric acid insoluble in acetic acid, ammonia |
| −-18.0·10−6 cm3/mol | |
Refractive index (nD) |
2.31 |
| Structure | |
| monoclinic | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Carcinogen and highly toxic |
| GHS labelling: | |
   | |
| Danger | |
| H350, H360, H373, H410 | |
| P201, P273, P308+P313, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>12 g/kg (mouse, oral) |
| Safety data sheet (SDS) | ICSC 0003 Sigma-Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
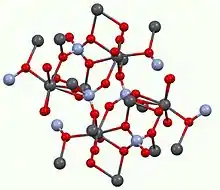
Lead chromate adopts the monazite structure, meaning that the connectivity of the atoms is very similar to other compounds of the type MM'O4. Pb(II) has a distorted coordination sphere being surrounded by eight oxides with Pb-O distances ranging from 2.53 to 2.80 Å. The chromate anion is tetrahedral, as usual.[2] Unstable polymorphs of lead chromate are the greenish yellow orthorhombic form and a red-orange tetragonal form.[1]
Applications
Approximately 37,000 tons were produced in 1996. The main applications are as a pigment in paints, under the name chrome yellow.[5]
Preparation
Lead(II) chromate can be produced by treating sodium chromate with lead salts such as lead(II) nitrate or by combining lead(II) oxide with chromic acid.
Related lead sulfochromate pigments are produced by the replacement of some chromate by sulfate, resulting in a mixed lead-chromate-sulfate compositions Pb(CrO4)1-x(SO4)x. This replacement is possible because sulfate and chromate are isostructural. Since sulfate is colorless, sulfochromates with high values of x are less intensely colored than lead chromate.[5] In some cases, chromate is replaced by molybdate.[1]
Reactions
Heating in hydroxide solution produces chrome red, a red or orange powder made by PbO and CrO3. Also, in hydroxide solution lead chromate slowly dissolves forming plumbite complex.
- PbCrO4 + 4 OH− → [Pb(OH)4]2− + CrO42−
Safety hazards
Despite containing both lead and hexavalent chromium, lead chromate is not particularly toxic because of its very low solubility. The LD50 for rats is only 5,000 mg/kg. Lead chromate is treated with great care in its manufacture, the main concerns being dust of the chromate precursor. "Extensive epidemiological investigations have given no indication that the practically insoluble lead chromate pigments have any carcinogenic properties".[5] Despite its low acute toxicity and lack of broad evidence for carcinogenicity, lead chromate is highly regulated in advanced countries. One of the greatest threats comes from inhalation of particles, so much effort has been devoted to production of low-dust forms of the pigment.[1]
In the 1800s, the product was used to impart a bright yellow color to some types of candy.[6] It is used (illegally) to enhance the color of certain spices, particularly turmeric,[7][8] particularly in Bangladesh.[9][10]
Unlike other lead-based paint pigments, lead chromate is still widely used.
References
- Erkens, LJH; Hamers, H.; Hermans, RJM; Claeys, E.; Bijnens, M. (2001). "Lead chromates: A Review of the State of the Art in 2000". Surface Coatings International Part B: Coatings Transactions. 84 (3): 169–176. doi:10.1007/BF02700395. S2CID 94606296.
- Quareni, S.; de Pieri, R. "A three-dimensional refinement of the structure of crocoite, PbCrO4" Acta Crystallographica 1965, volume 19, p287-p289. doi:10.1107/S0365110X65003304
- "Sunflowers - Van Gogh Museum". vangoghmuseum.nl. Archived from the original on 29 October 2016. Retrieved 21 September 2016.
- Monico, Letizia; Janssens, Koen; Hendriks, Ella; Vanmeert, Frederik; Van Der Snickt, Geert; Cotte, Marine; Falkenberg, Gerald; Brunetti, Brunetto Giovanni; Miliani, Costanza (2015). "Evidence for Degradation of the Chrome Yellows in Van Gogh's Sunflowers: A Study Using Noninvasive In Situ Methods and Synchrotron-Radiation-Based X-ray Techniques". Angewandte Chemie International Edition. 54 (47): 13923–13927. doi:10.1002/anie.201505840. PMID 26482035. S2CID 2268072.
- Völz, Hans G.; et al. (2006). "Pigments, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_243.pub2.
- Wisconsin. State Board of Health (1887). Progress Report of Public Health in Wisconsin, Volume 10. p. 92. Retrieved 17 July 2013. (Google Books)
- "The American Spice Trade Association's Statement on Lead in Turmeric - ASTA: The Voice of the U.S. Spice Industry in the Global Market". ASTA: The Voice of the U.S. Spice Industry in the Global Market. 28 October 2013. Retrieved 21 November 2018.
- Angelon-Gaetz, Kim A.; Klaus, Christen; Chaudhry, Ezan A.; Bean, Deidre K. (23 November 2018). "Lead in Spices, Herbal Remedies, and Ceremonial Powders Sampled from Home Investigations for Children with Elevated Blood Lead Levels — North Carolina, 2011–2018". MMWR. Morbidity and Mortality Weekly Report. 67 (46): 1290–1294. doi:10.15585/mmwr.mm6746a2. ISSN 0149-2195. PMC 6289082. PMID 30462630.
- "Researchers find lead in turmeric". Phys. Stanford University. 24 September 2019. Retrieved 1 October 2019.
- Forsyth, Jenna E.; Nurunnahar, Syeda; Islam, Sheikh Shariful; Baker, Musa; Yeasmin, Dalia; Islam, M. Saiful; Rahman, Mahbubur; Fendorf, Scott; Ardoin, Nicole M.; Winch, Peter J.; Luby, Stephen P. (December 2019). "Turmeric means "yellow" in Bengali: Lead chromate pigments added to turmeric threaten public health across Bangladesh". Environmental Research. 179 (Pt A): 108722. Bibcode:2019ER....179j8722F. doi:10.1016/j.envres.2019.108722. PMID 31550596.

