Limelight
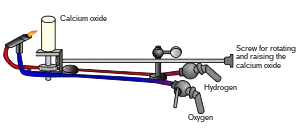
Limelight (also known as Drummond light or calcium light)[1] is a type of stage lighting once used in theatres and music halls. An intense illumination is created when a flame fed by oxygen and hydrogen is directed at a cylinder of quicklime (calcium oxide),[2] which can be heated to 2,572 °C (4,662 °F) before melting. The light is produced by a combination of incandescence and candoluminescence. Although it has long since been replaced by electric lighting, the term has nonetheless survived, as someone in the public eye is still said to be "in the limelight". The actual lamps are called "limes", a term which has been transferred to electrical equivalents.
History
Discovery and invention
The limelight effect was discovered in the 1820s by Goldsworthy Gurney,[3][4] based on his work with the "oxy-hydrogen blowpipe", credit for which is normally given to Robert Hare. In 1825, a Scottish engineer, Thomas Drummond (1797–1840), saw a demonstration of the effect by Michael Faraday[5] and realized that the light would be useful for surveying. Drummond built a working version in 1826, and the device is sometimes called the Drummond light after him.
Early use in the United Kingdom
The earliest known use of limelight at a public performance was outdoors, over Herne Bay Pier, Kent, on the night of 3 October 1836 to illuminate a juggling performance by magician Ching Lau Lauro. This performance was part of the celebrations following the laying of the foundation stone of the Clock Tower. The advertising leaflet called it koniaphostic light and announced that "the whole pier is overwhelmed with a flood of beautiful white light".[6][7] Limelight was first used for indoor stage illumination in the Covent Garden Theatre in London in 1837 and enjoyed widespread use in theatres around the world in the 1860s and 1870s.[8] Limelights were employed to highlight solo performers in the same manner as modern spotlights.[9]
Early use in the United States
During the American Civil War in July and August 1863 calcium lights were used during the siege of Fort Wagner, allowing Union forces to illuminate their artillery target at night while simultaneously blinding Confederate gunners and riflemen. Calcium lights were also installed on Union Navy ships.[10]
Limelight was replaced by electric arc lighting in the late 19th century.
Gallery
 Heating calcium hydroxide (slaked lime) in a stove burner causes it to glow, although this is not as bright as a real limelight
Heating calcium hydroxide (slaked lime) in a stove burner causes it to glow, although this is not as bright as a real limelight.jpg.webp) 19th century limelight used for stage lighting (collection: Stadsschouwburg (city theatre) Bruges, Belgium)
19th century limelight used for stage lighting (collection: Stadsschouwburg (city theatre) Bruges, Belgium)
See also
References
- James R. Smith (2004). San Francisco's Lost Landmarks, Quill Driver Books.
- "Chemical of the Week – Lime". scifun.chem.wisc.edu. Archived from the original on 17 February 2008. Retrieved 24 December 2017.
- Limelight – Leeds University Archived 19 February 2011 at the Wayback Machine, Retrieved 18 October 2013.
- Faraday, Michael; James, Frank A. J. L (1999). The Correspondence of Michael Faraday. p. 11. ISBN 978-0-86341-251-6.
- Carver, Craig M. (1991). A history of English in its own words. New York: HarperCollins. p. 158. ISBN 0-06-270013-8.
- Bundock 2000, p. 6.
- The Mechanic and Chemist: A Magazine of the Arts and Sciences. 1839. p. 354.
- Almqvist, Ebbe (2003). History of industrial gases. Springer Science & Business Media. pp. 72–73. ISBN 978-0-306-47277-0.
- Reid, Francis (2001). The Stage Lighting Handbook (Stage and Costume) (6 Rev ed.). UK: A & C Black Publishers Ltd. ISBN 0-7136-5396-5.
- In the Limelight: A Civil War Military Innovation
Bibliography
- Bundock, Mike (2000). Herne Bay Clock Tower: A Descriptive History. Herne Bay: Pierhead Publications. ISBN 978-0953897704.
- Art Gallery of South Australia History of Limelights
External links
![]() Media related to Limelight at Wikimedia Commons
Media related to Limelight at Wikimedia Commons

.jpg.webp)
