Lumican
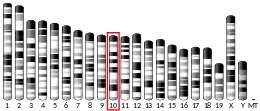
Lumican,[5] also known as LUM, is an extracellular matrix protein that, in humans, is encoded by the LUM gene on chromosome 12.[6][7]
Structure
Lumican is a proteoglycan Class II member of the small leucine-rich proteoglycan (SLRP) family that includes decorin, biglycan, fibromodulin, keratocan, epiphycan, and osteoglycin.[8]
Like the other SLRPs, lumican has a molecular weight of about 40 kiloDaltons and has four major intramolecular domains:[9]
- a signal peptide of 16 amino acid residues;
- a negatively-charged N-terminal domain containing sulfated tyrosine and disulfide bond(s);
- ten tandem leucine-rich repeats allowing lumican to bind to other extracellular components such as collagen;
- a carboxyl terminal domain of 50 amino acid residues containing two conserved cysteines 32 residues apart.
There are four N-linked sites within the leucine-rich repeat domain of the protein core that can be substituted with keratan sulfate. The core protein of lumican (like decorin and fibromodulin) is horseshoe shaped. This enables it bind to collagen molecules within a collagen fibril, thus helping keep adjacent fibrils apart.[10]
Function
Lumican is a major keratan sulfate proteoglycan of the cornea but is ubiquitously distributed in most mesenchymal tissues throughout the body.[11] Lumican is involved in collagen fibril organization and circumferential growth, corneal transparency, and epithelial cell migration and tissue repair.[6] Corneal transparency is possible due to the exact alignment of collagen fibers by lumican (and keratocan) in the intrafibrillar space.
Clinical significance
Mice that have the lumican gene knocked out (Lum-/-) develop opacities of the cornea in both eyes and fragile skin.[12] The lumican (LUM) gene was thought to be a candidate susceptibility gene for high myopia; however, a meta-analysis showed no association between LUM polymorphism and high myopia susceptibility in all genetic models studied.[13]
Lum knockout mice also have abnormal collagen in their heart tissue, with fewer and thicker fibrils.[14] Mice deficient in both lumican and fibromodulin develop severe tendinopathy (tendon pathology), revealing the importance of these SLRPs in the development of correctly sized and aligned collagen fibers in tendon.[15] Along with other extracellular matrix components, lumican expression was increased in equine flexor tendons six weeks after an injury.[16]
Lumican is present in the extracellular matrix of uteral tissues in fertile women.[17] There is an increase of lumican during the proliferative to secretory phase of the endometrium. In menopausal endometrial tissue, the level of lumican expression decreases and is also low in pathological compared to normal endometrium.
Lumican is highly expressed in pleural effusions (lung fluid) of patients with adenocarcinoma.[18] Its expression was low in cancer cells but high in the extracellular matrix surrounding the tumor. Lumican expression was not associated with tumor grade or stage. In about half the patients with pancreatic ductal adenocarcinoma tested,[19] lumican in the extracellular matrix around the tumor was associated with a reduction in metastatic recurrence after surgery and with a three-fold longer survival than patients without stromal lumican. As lumican can directly bind to and inhibit matrix metalloproteinase-14 (MMP14), lumican may limit tumor progression by preventing extracellular matrix collagen proteolysis by this enzyme.[20]
References
- GRCh38: Ensembl release 89: ENSG00000139329 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000036446 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Blochberger, T. C.; Vergnes, J. P.; Hempel, J.; Hassell, J. R. (1992-01-05). "cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine". The Journal of Biological Chemistry. 267 (1): 347–352. doi:10.1016/S0021-9258(18)48500-6. ISSN 0021-9258. PMID 1370446.
- "Entrez Gene: LUM lumican".
- Chakravarti S, Stallings RL, SundarRaj N, Cornuet PK, Hassell JR (Jun 1995). "Primary structure of human lumican (keratan sulfate proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3-q22". Genomics. 27 (3): 481–8. doi:10.1006/geno.1995.1080. PMID 7558030.
- Iozzo RV, Schaefer L (Mar 2015). "Proteoglycan form and function: A comprehensive nomenclature of proteoglycans". Matrix Biology. 42: 11–55. doi:10.1016/j.matbio.2015.02.003. PMC 4859157. PMID 25701227.
- Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW (Jan 2006). "Focus on molecules: lumican". Experimental Eye Research. 82 (1): 3–4. doi:10.1016/j.exer.2005.08.012. PMC 2876311. PMID 16213485.
- Scott JE (1996). "Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen". Biochemistry. 35 (27): 8795–9. doi:10.1021/bi960773t. PMID 8688414.
- Chakravarti S (2002). "Functions of lumican and fibromodulin: lessons from knockout mice". Glycoconjugate Journal. 19 (4–5): 287–93. doi:10.1023/A:1025348417078. PMID 12975607. S2CID 3636866.
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H (Jun 1998). "Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican". The Journal of Cell Biology. 141 (5): 1277–86. doi:10.1083/jcb.141.5.1277. PMC 2137175. PMID 9606218.
- Li M, Zhai L, Zeng S, Peng Q, Wang J, Deng Y, Xie L, He Y, Li T (Jul 2014). "Lack of association between LUM rs3759223 polymorphism and high myopia". Optometry and Vision Science. 91 (7): 707–12. doi:10.1097/OPX.0000000000000302. PMID 24927138. S2CID 27647081.
- Mienaltowski MJ, Birk DE (2014). "Mouse models in tendon and ligament research". Progress in Heritable Soft Connective Tissue Diseases. Advances in Experimental Medicine and Biology. Vol. 802. pp. 201–30. doi:10.1007/978-94-007-7893-1_13. ISBN 978-94-007-7892-4. PMID 24443029.
- Dupuis LE, Berger MG, Feldman S, Doucette L, Fowlkes V, Chakravarti S, Thibaudeau S, Alcala NE, Bradshaw AD, Kern CB (Apr 2015). "Lumican deficiency results in cardiomyocyte hypertrophy with altered collagen assembly". Journal of Molecular and Cellular Cardiology. 84: 70–80. doi:10.1016/j.yjmcc.2015.04.007. PMC 4468017. PMID 25886697.
- Jacobson E, Dart AJ, Mondori T, Horadogoda N, Jeffcott LB, Little CB, Smith MM (2015). "Focal experimental injury leads to widespread gene expression and histologic changes in equine flexor tendons". PLOS ONE. 10 (4): e0122220. Bibcode:2015PLoSO..1022220J. doi:10.1371/journal.pone.0122220. PMC 4383631. PMID 25837713.
- Lucariello A, Trabucco E, Boccia O, Perna A, Sellitto C, Castaldi MA, De Falco M, De Luca A, Cobellis L (2015). "Small leucine rich proteoglycans are differently distributed in normal and pathological endometrium". In Vivo. 29 (2): 217–22. PMID 25792648.
- Cappellesso R, Millioni R, Arrigoni G, Simonato F, Caroccia B, Iori E, Guzzardo V, Ventura L, Tessari P, Fassina A (2015). "Lumican is overexpressed in lung adenocarcinoma pleural effusions". PLOS ONE. 10 (5): e0126458. Bibcode:2015PLoSO..1026458C. doi:10.1371/journal.pone.0126458. PMC 4427354. PMID 25961303.
- Li X, Truty MA, Kang Y, Chopin-Laly X, Zhang R, Roife D, Chatterjee D, Lin E, Thomas RM, Wang H, Katz MH, Fleming JB (Dec 2014). "Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery". Clinical Cancer Research. 20 (24): 6529–40. doi:10.1158/1078-0432.CCR-14-0970. PMC 4268437. PMID 25336691.
- Pietraszek K, Chatron-Colliet A, Brézillon S, Perreau C, Jakubiak-Augustyn A, Krotkiewski H, Maquart FX, Wegrowski Y (Nov 2014). "Lumican: a new inhibitor of matrix metalloproteinase-14 activity". FEBS Letters. 588 (23): 4319–24. doi:10.1016/j.febslet.2014.09.040. PMID 25304424. S2CID 36035579.
Further reading
- Grover J, Chen XN, Korenberg JR, Roughley PJ (Sep 1995). "The human lumican gene. Organization, chromosomal location, and expression in articular cartilage". The Journal of Biological Chemistry. 270 (37): 21942–9. doi:10.1074/jbc.270.37.21942. PMID 7665616.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Rada JA, Cornuet PK, Hassell JR (Jun 1993). "Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins". Experimental Eye Research. 56 (6): 635–48. doi:10.1006/exer.1993.1081. PMID 8595806.
- Hillier LD, Lennon G, Becker M, Bonaldo MF, Chiapelli B, Chissoe S, Dietrich N, DuBuque T, Favello A, Gish W, Hawkins M, Hultman M, Kucaba T, Lacy M, Le M, Le N, Mardis E, Moore B, Morris M, Parsons J, Prange C, Rifkin L, Rohlfing T, Schellenberg K, Bento Soares M, Tan F, Thierry-Meg J, Trevaskis E, Underwood K, Wohldman P, Waterston R, Wilson R, Marra M (Sep 1996). "Generation and analysis of 280,000 human expressed sequence tags". Genome Research. 6 (9): 807–828. doi:10.1101/gr.6.9.807. PMID 8889549.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Matsushima N, Ohyanagi T, Tanaka T, Kretsinger RH (Feb 2000). "Super-motifs and evolution of tandem leucine-rich repeats within the small proteoglycans--biglycan, decorin, lumican, fibromodulin, PRELP, keratocan, osteoadherin, epiphycan, and osteoglycin". Proteins. 38 (2): 210–25. doi:10.1002/(SICI)1097-0134(20000201)38:2<210::AID-PROT9>3.0.CO;2-1. PMID 10656267. S2CID 39643243.
- Svensson L, Närlid I, Oldberg A (Mar 2000). "Fibromodulin and lumican bind to the same region on collagen type I fibrils". FEBS Letters. 470 (2): 178–82. doi:10.1016/S0014-5793(00)01314-4. PMID 10734230. S2CID 46539089.
- Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivelä T, Kucherlapati R, Forsius H, de la Chapelle A (May 2000). "Mutations in KERA, encoding keratocan, cause cornea plana". Nature Genetics. 25 (1): 91–5. doi:10.1038/75664. PMID 10802664. S2CID 8837115.
- Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR (May 2000). "Independent modulation of collagen fibrillogenesis by decorin and lumican". Cellular and Molecular Life Sciences. 57 (5): 859–63. doi:10.1007/s000180050048. PMID 10892350. S2CID 21998935.
- Grover J, Liu CY, Kao WW, Roughley PJ (Dec 2000). "Analysis of the human lumican gene promoter". The Journal of Biological Chemistry. 275 (52): 40967–73. doi:10.1074/jbc.M004134200. PMID 11016924.
- Naito Z, Ishiwata T, Kurban G, Teduka K, Kawamoto Y, Kawahara K, Sugisaki Y (May 2002). "Expression and accumulation of lumican protein in uterine cervical cancer cells at the periphery of cancer nests". International Journal of Oncology. 20 (5): 943–8. doi:10.3892/ijo.20.5.943. PMID 11956587.
- Lu YP, Ishiwata T, Kawahara K, Watanabe M, Naito Z, Moriyama Y, Sugisaki Y, Asano G (Aug 2002). "Expression of lumican in human colorectal cancer cells". Pathology International. 52 (8): 519–26. doi:10.1046/j.1440-1827.2002.01384.x. PMID 12366811. S2CID 38324942.
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA (Dec 2002). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proceedings of the National Academy of Sciences of the United States of America. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, Veenstra TD, Adkins JN, Pounds JG, Fagan R, Lobley A (Apr 2004). "The human plasma proteome: a nonredundant list developed by combination of four separate sources". Molecular & Cellular Proteomics. 3 (4): 311–26. doi:10.1074/mcp.M300127-MCP200. PMID 14718574.
- Bunkenborg J, Pilch BJ, Podtelejnikov AV, Wiśniewski JR (Feb 2004). "Screening for N-glycosylated proteins by liquid chromatography mass spectrometry". Proteomics. 4 (2): 454–65. doi:10.1002/pmic.200300556. PMID 14760718. S2CID 29261009.
- Botella LM, Sanz-Rodriguez F, Sanchez-Elsner T, Langa C, Ramirez JR, Vary C, Roughley PJ, Bernabeu C (Jan 2004). "Lumican is down-regulated in cells expressing endoglin. Evidence for an inverse correlationship between Endoglin and Lumican expression". Matrix Biology. 22 (7): 561–72. doi:10.1016/j.matbio.2003.11.006. PMID 14996436.
- Vuillermoz B, Khoruzhenko A, D'Onofrio MF, Ramont L, Venteo L, Perreau C, Antonicelli F, Maquart FX, Wegrowski Y (Jun 2004). "The small leucine-rich proteoglycan lumican inhibits melanoma progression". Experimental Cell Research. 296 (2): 294–306. doi:10.1016/j.yexcr.2004.02.005. PMID 15149859.
- Köninger J, Giese T, di Mola FF, Wente MN, Esposito I, Bachem MG, Giese NA, Büchler MW, Friess H (Sep 2004). "Pancreatic tumor cells influence the composition of the extracellular matrix". Biochemical and Biophysical Research Communications. 322 (3): 943–9. doi:10.1016/j.bbrc.2004.08.008. PMID 15336555.
- Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H, Takino T, Sato H (Oct 2004). "Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar". Cancer Research. 64 (19): 7058–64. doi:10.1158/0008-5472.CAN-04-1038. PMID 15466200.