Lupus nephritis
Lupus nephritis is an inflammation of the kidneys caused by systemic lupus erythematosus (SLE), an autoimmune disease.[3] It is a type of glomerulonephritis in which the glomeruli become inflamed. Since it is a result of SLE, this type of glomerulonephritis is said to be secondary, and has a different pattern and outcome from conditions with a primary cause originating in the kidney.[4][2] The diagnosis of lupus nephritis depends on blood tests, urinalysis, X-rays, ultrasound scans of the kidneys, and a kidney biopsy. On urinalysis, a nephritic picture is found and red blood cell casts, red blood cells and proteinuria is found.
| Lupus nephritis | |
|---|---|
| Other names | SLE nephritis[1] |
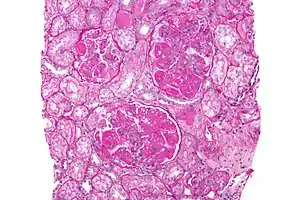 | |
| Micrograph of diffuse proliferative lupus nephritis showing increased mesangial matrix and mesangial hypercellularity. Kidney biopsy. PAS stain. | |
| Specialty | Nephrology |
| Symptoms | Joint pain or swelling[2] |
| Causes | Complication of systemic lupus erythematosus.[3] |
| Diagnostic method | Complement levels, Urinalysis[3] |
| Treatment | Corticosteroids may be used[3] |
Classification
The World Health Organization has divided lupus nephritis into five stages based on the biopsy. This classification was defined in 1982 and revised in 1995.[5][6] Class IV disease (Diffuse proliferative nephritis) is both the most severe, and the most common subtype. Class VI (advanced sclerosing lupus nephritis) is a final class which is included by most practitioners. It is thought to be due to the chronic interferon exposure.[7]
| Order | Name | Incidence[8] | Light microscopy | Electron microscopy | Clinical findings and other tests | Treatment |
|---|---|---|---|---|---|---|
| Class I | Minimal mesangial glomerulonephritis | 5% | Normal appearance | Mesangial deposits are visible under an electron microscope | Kidney failure is very rare in this form.[8] Normal urinalysis.[9] | |
| Class II | Mesangial proliferative glomerulonephritis | 20% | Mesangial hypercellularity and matrix expansion. | Microscopic haematuria with or without proteinuria may be seen. Hypertension, nephrotic syndrome, and acute kidney injury are very rare at this stage.[9] | Responds to high doses of corticosteroids | |
| Class III | Focal glomerulonephritis | 25% | Sclerotic lesions involving less than 50% of the glomeruli, which can be segmental or global, and active or chronic, with endocapillary or extracapillary proliferative lesions. | Subendothelial deposits are noted, and some mesangial changes may be present | Immunofluorescence reveals positively for IgG, IgA, IgM, C3, and C1q. Clinically, haematuria and proteinuria are present, with or without nephrotic syndrome, hypertension, and elevated serum creatinine.[9] | Often successfully responds to high doses of corticosteroids |
| Class IV | Diffuse proliferative nephritis | 40% | More than 50% of glomeruli are involved. Lesions can be segmental or global, and active or chronic, with endocapillary or extracapillary proliferative lesions. | Under electron microscopy, subendothelial deposits are noted, and some mesangial changes may be present. | Clinically, haematuria and proteinuria are present, frequently with nephrotic syndrome, hypertension, hypocomplementemia, elevated anti-dsDNA titres and elevated serum creatinine.[9] Kidney failure is common.[8] | Corticosteroids and immunosuppressant drugs |
| Class V | Membranous glomerulonephritis | 10% | Diffuse thickening of the glomerular capillary wall (segmentally or globally), with diffuse membrane thickening, and subepithelial deposits seen under the electron microscope. | Signs of nephrotic syndrome. Microscopic haematuria and hypertension may also be seen. Can also lead to thrombotic complications such as renal vein thromboses or pulmonary emboli.[9] Kidney failure is uncommon.[8] | ||
| Class VI | Advanced sclerosing lupus nephritis.[10] | Global sclerosis involving more than 90% of glomeruli, and represents healing of prior inflammatory injury. | Active glomerulonephritis is not usually present. This stage is characterised by slowly progressive kidney dysfunction, with relatively bland urine sediment. | Response to immunotherapy is usually poor. |
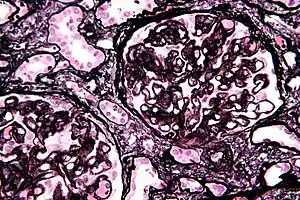
A tubuloreticular inclusion within capillary endothelial cells is also characteristic of lupus nephritis and can be seen under an electron microscope in all stages. It is not diagnostic however, as it exists in other conditions such as HIV infection.[11]
Signs and symptoms
Cause
The cause of lupus nephritis, a genetic predisposition, plays a significant role in lupus nephritis. Multiple genes, many of which are not yet identified, mediate this genetic predisposition.[10][13]
The immune system protects the human body from infection, and with immune system problems it cannot distinguish between harmful and healthy substances. Lupus nephritis affects approximately 3 out of 10,000 people.[3]
Pathophysiology
The pathophysiology of lupus nephritis has autoimmunity contributing significantly. Autoantibodies direct themselves against nuclear elements. The characteristics of nephritogenic autoantibodies (lupus nephritis) are antigen specificity directed at nucleosome, high affinity autoantibodies form intravascular immune complexes, and autoantibodies of certain isotypes activate complement.[10]
Treatment
Drug regimens prescribed for lupus nephritis include mycophenolate mofetil (MMF), intravenous cyclophosphamide with corticosteroids, and the immune suppressant azathioprine with corticosteroids.[14] MMF and cyclophosphamide with corticosteroids are equally effective in achieving remission of the disease, however the results of a recent systematic review found that immunosuppressive drugs were better than corticosteroids for renal outcomes.[15] MMF is safer than cyclophosphamide with corticosteroids, with less chance of causing ovarian failure, immune problems or hair loss. It also works better than azathioprine with corticosteroids for maintenance therapy.[16][17] A 2016 network meta-analysis, which included 32 RCTs of lupus nephritis, demonstrated that tacrolimus and MMF followed by azathioprine maintenance were associated with a lower risk of serious infection when compared to other immunosuppressants or glucocorticoids.[18][19] Individuals with lupus nephritis have a high risk for B-cell lymphoma (which begins in the immune system cells).[2]
Prognosis
In those who have SLE, concomitant lupus nephritis is associated with a worse overall prognosis.[20] 10-30% of people with lupus nephritis progress to kidney failure requiring dialysis, with the 5 year mortality rate of lupus nephritis being 5-25%.[20] The proliferative forms of lupus nephritis are associated with a higher risk of progression to end stage kidney disease.[20] Black and Hispanic people with lupus nephritis are more likely to present with severe disease at initial presentation (with more proteinuria and more extensive histopathologic changes) and progress to end stage kidney disease. This is thought to be due to socioeconomic factors but auto-antibodies strongly associated with lupus nephritis such as anti-Sm, anti-Ro and anti-ribonucleoprotein are also more commonly seen in Black and Hispanic people.[20] Men with SLE tend to have more aggressive forms of lupus nephritis as well with a higher risk of progression to end stage kidney disease and higher risk of concurrent cardiovascular disease.[20]
See also
References
- Ponticelli, C.; Moroni, G. (2005-01-01). "Renal transplantation in lupus nephritis". Lupus. 14 (1): 95–98. doi:10.1191/0961203305lu2067oa. ISSN 0961-2033. PMID 15732296. S2CID 36968488.
- "Lupus Nephritis". www.niddk.nih.gov. Retrieved 2015-10-31.
- "Lupus nephritis: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2015-10-31.
- Saxena, Ramesh; Mahajan, Tina; Mohan, Chandra (2011-01-01). "Lupus nephritis: current update". Arthritis Research & Therapy. 13 (5): 240. doi:10.1186/ar3378. ISSN 1478-6354. PMC 3308062. PMID 22078716.
- Weening JJ, D'Agati VD, Schwartz MM, et al. (February 2004). "The classification of glomerulonephritis in systemic lupus erythematosus revisited". J. Am. Soc. Nephrol. 15 (2): 241–50. doi:10.1097/01.ASN.0000108969.21691.5D. PMID 14747370.
- "National Guideline Clearinghouse | American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis". www.guideline.gov. Archived from the original on 2015-09-18. Retrieved 2015-11-01.
- Karageorgas TP, Tseronis DD, Mavragani CP (2011). "Activation of type I interferon pathway in systemic lupus erythematosus: association with distinct clinical phenotypes". Journal of Biomedicine & Biotechnology. 2011: 1–13. doi:10.1155/2011/273907. PMC 3227532. PMID 22162633.
- Table 6-4 in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-7153-5.
- Lewis, Edmund J.; Schwartz, Melvin M. (2010-11-04). Lupus Nephritis. OUP Oxford. pp. 174–177. ISBN 9780199568055.
- "Lupus Nephritis: Practice Essentials, Background, Pathophysiology". 2018-12-23.
{{cite journal}}: Cite journal requires|journal=(help) - Kfoury H (2014). "Tubulo-reticular inclusions in lupus nephritis: are they relevant?". Saudi Journal of Kidney Diseases and Transplantation. 25 (3): 539–43. doi:10.4103/1319-2442.132169. PMID 24821149.
- "Lupus Nephritis - National Library of Medicine". PubMed Health. Retrieved 2015-11-03.
- Salgado, Alberto (2012). "Lupus Nephritis: An Overview of Recent Findings". Autoimmune Diseases. 2012: 849684. doi:10.1155/2012/849684. PMC 3318208. PMID 22536486.
- Singh, Jasvinder A.; Hossain, Alomgir; Kotb, Ahmed; Wells, George A. (2016). "Comparative effectiveness of immunosuppressive drugs and corticosteroids for lupus nephritis: a systematic review and network meta-analysis". Systematic Reviews. 5 (1): 155. doi:10.1186/s13643-016-0328-z. ISSN 2046-4053. PMC 5020478. PMID 27619512.
- Singh, Jasvinder A.; Hossain, Alomgir; Kotb, Ahmed; Oliveira, Ana; Mudano, Amy S.; Grossman, Jennifer; Winthrop, Kevin; Wells, George A. (2016). "Treatments for Lupus Nephritis: A Systematic Review and Network Metaanalysis". The Journal of Rheumatology. 43 (10): 1801–1815. doi:10.3899/jrheum.160041. ISSN 0315-162X. PMID 27585688.
- Tunnicliffe, David J.; Palmer, Suetonia C.; Henderson, Lorna; Masson, Philip; Craig, Jonathan C.; Tong, Allison; Singh-Grewal, Davinder; Flanc, Robert S.; Roberts, Matthew A.; Webster, Angela C.; Strippoli, Giovanni Fm (29 June 2018). "Immunosuppressive treatment for proliferative lupus nephritis". The Cochrane Database of Systematic Reviews. 2018 (6): CD002922. doi:10.1002/14651858.CD002922.pub4. ISSN 1469-493X. PMC 6513226. PMID 29957821.
- Masson, Philip (2011). "Induction and maintenance treatment of proliferative lupus nephritis" (PDF). Nephrology. 18: 71–72. doi:10.1111/nep.12011. S2CID 56952099. Retrieved 4 November 2015.
- Singh, Jasvinder A.; Hossain, Alomgir; Kotb, Ahmed; Wells, George (2016-09-13). "Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis". BMC Medicine. 14 (1): 137. doi:10.1186/s12916-016-0673-8. ISSN 1741-7015. PMC 5022202. PMID 27623861.
- Tang, Kuo-Tung; Tseng, Chien-Hua; Hsieh, Tsu-Yi; Chen, Der-Yuan (June 2018). "Induction therapy for membranous lupus nephritis: a systematic review and network meta-analysis". International Journal of Rheumatic Diseases. 21 (6): 1163–1172. doi:10.1111/1756-185X.13321. ISSN 1756-185X. PMID 29879319. S2CID 46951249.
- Parikh, Samir V.; Almaani, Salem; Brodsky, Sergey; Rovin, Brad H. (1 August 2020). "Update on Lupus Nephritis: Core Curriculum 2020". American Journal of Kidney Diseases. 76 (2): 265–281. doi:10.1053/j.ajkd.2019.10.017. ISSN 0272-6386. PMID 32220510. Retrieved 22 July 2021.
Further reading
- Lahita, Robert G. (2004-06-09). Systemic Lupus Erythematosus. Academic Press. ISBN 9780080474540.
- Greenberg, Arthur; Cheung, Alfred K. (2005-01-01). Primer on Kidney Diseases. Elsevier Health Sciences. ISBN 978-1416023128.
- Castro-Santana, Lesliane E.; Colón, Marilú; Molina, María J.; Rodríguez, Vanessa E.; Mayor, Angel M.; Vilá, Luis M. (2010-01-01). "Efficacy of two cyclophosphamide regimens for the treatment of lupus nephritis in Puerto Ricans: low versus standard dose". Ethnicity & Disease. 20 (1): S1–116–21. ISSN 1049-510X. PMC 3572835. PMID 20521398.
- Appel, Gerald B.; Contreras, Gabriel; Dooley, Mary Anne; Ginzler, Ellen M.; Isenberg, David; Jayne, David; Li, Lei-Shi; Mysler, Eduardo; Sánchez-Guerrero, Jorge (2009-05-01). "Mycophenolate Mofetil versus Cyclophosphamide for Induction Treatment of Lupus Nephritis". Journal of the American Society of Nephrology. 20 (5): 1103–1112. doi:10.1681/ASN.2008101028. ISSN 1046-6673. PMC 2678035. PMID 19369404.