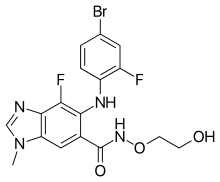
Binimetinib
Binimetinib, sold under the brand name Mektovi, is an anti-cancer medication used to treat various cancers.[3] Binimetinib is a selective inhibitor of MEK, a central kinase in the tumor-promoting MAPK pathway.[4] Inappropriate activation of the pathway has been shown to occur in many cancers.[4] In June 2018 it was approved by the FDA in combination with encorafenib for the treatment of patients with unresectable or metastatic BRAF V600E or V600K mutation-positive melanoma.[5][6] In October 2023, it was approved by the FDA for treatment of NSCLC with a BRAF V600E mutation in combination with encorafenib .[7] It was developed by Array Biopharma.
 | |
| Clinical data | |
|---|---|
| Trade names | Mektovi |
| Other names | MEK162, ARRY-162, ARRY-438162 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618041 |
| License data |
|
| Drug class | Antineoplastic Agents |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.617 |
| Chemical and physical data | |
| Formula | C17H15BrF2N4O3 |
| Molar mass | 441.233 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mechanism of action
Binimetinib is an orally available inhibitor of mitogen-activated protein kinase kinase (MEK), or more specifically, a MAP2K inhibitor.[8] MEK is part of the RAS pathway, which is involved in cell proliferation and survival. MEK is upregulated in many forms of cancer.[9] Binimetinib, uncompetitive with ATP, binds to and inhibits the activity of MEK1/2 kinase, which has been shown to regulate several key cellular activities including proliferation, survival, and angiogenesis.[10] MEK1/2 are dual-specificity threonine/tyrosine kinases that play key roles in the activation of the RAS/RAF/MEK/ERK pathway and are often upregulated in a variety of tumor cell types.[11] Inhibition of MEK1/2 prevents the activation of MEK1/2 dependent effector proteins and transcription factors, which may result in the inhibition of growth factor-mediated cell signaling.[12] As demonstrated in preclinical studies, this may eventually lead to an inhibition of tumor cell proliferation and an inhibition in production of various inflammatory cytokines including interleukin-1, -6 and tumor necrosis factor.[12]
Development
In 2015, it was in phase III clinical trials for ovarian cancer,[13] BRAF mutant melanoma,[14] and NRAS Q61 mutant melanoma.[15]
In December 2015, the company announced that the mutant-NRAS melanoma trial was successful.[16] In the trial, those receiving binimetinib had a median progression-free survival of 2.8 months versus 1.5 months for those on the standard dacarbazine treatment.[17] NDA submitted Jun 2016,[18] and the FDA should decide by 30 June 2017.[19]
In April 2016, it was reported that the phase III trial for low-grade ovarian cancer was terminated due to lack of efficacy.[20]
Binimetinib was studied for treatment of rheumatoid arthritis, but a phase II trial did not show benefit.
In 2017, the FDA informed Array Biopharma that the phase III trial data was not sufficient and the New Drug Application was withdrawn.[21]
In June 2018, it was approved for the treatment of certain melanomas by the U.S. Food and Drug Administration (FDA) in combination with encorafenib.[5] The FDA approved binimetinib based primarily on evidence from one clinical trial (NCT01909453) of 383 patients with BRAF V600 mutation-positive melanoma that was advanced or could not be removed by surgery.[6] The trial was conducted at 162 sites in Europe, North America, and various countries around the world.[6]
In October 2023, the US Food and Drug Administration approved encorafenib with binimetinib for adults with metastatic non-small cell lung cancer (NSCLC) with a BRAF V600E mutation, as detected by an FDA-approved test.[7]
References
- "Product monograph" (PDF). hres.ca. Retrieved 19 October 2023.
- "Summary Basis of Decision (SBD) for Mektovi". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- "Binimetinib". Array Biopharma.
- Koelblinger P, Dornbierer J, Dummer R (August 2017). "A review of binimetinib for the treatment of mutant cutaneous melanoma". Future Oncology. 13 (20): 1755–1766. doi:10.2217/fon-2017-0170. PMID 28587477.
- "FDA approves encorafenib and binimetinib in combination for unresectable or metastatic melanoma with BRAF mutations" (Press release). U.S. Food and Drug Administration (FDA). 27 June 2018. Archived from the original on 19 December 2019. Retrieved 17 July 2018.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Drug Trial Snapshot: Mektovi". U.S. Food and Drug Administration (FDA). 19 December 2019. Archived from the original on 19 December 2019. Retrieved 19 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves encorafenib with binimetinib for metastatic non-small cell lung cancer with a BRAF V600E mutation". U.S. Food and Drug Administration. 11 October 2023. Retrieved 11 October 2023.
- Wu PK, Park JI (December 2015). "MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms". Seminars in Oncology. 42 (6): 849–62. doi:10.1053/j.seminoncol.2015.09.023. PMC 4663016. PMID 26615130.
- "Binimetinib". PubChem.
- Ascierto PA, Schadendorf D, Berking C, et al. (March 2013). "MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study". The Lancet. Oncology. 14 (3): 249–56. doi:10.1016/S1470-2045(13)70024-X. PMID 23414587.
- Mehdizadeh A, Somi MH, Darabi M, et al. (February 2016). "Extracellular signal-regulated kinase 1 and 2 in cancer therapy: a focus on hepatocellular carcinoma". Molecular Biology Reports. 43 (2): 107–16. doi:10.1007/s11033-016-3943-9. PMID 26767647. S2CID 15113957.
- Woodfield SE, Zhang L, Scorsone KA, et al. (March 2016). "Binimetinib inhibits MEK and is effective against neuroblastoma tumor cells with low NF1 expression". BMC Cancer. 16: 172. doi:10.1186/s12885-016-2199-z. PMC 4772351. PMID 26925841.
- Clinical trial number NCT01849874 for "A Study of MEK162 vs. Physician's Choice Chemotherapy in Patients With Low-grade Serous Ovarian, Fallopian Tube or Peritoneal Cancer" at ClinicalTrials.gov
- Clinical trial number NCT01909453 for "Study Comparing Combination of LGX818 Plus MEK162 Versus Vemurafenib and LGX818 Monotherapy in BRAF Mutant Melanoma (COLUMBUS)" at ClinicalTrials.gov
- Clinical trial number NCT01763164 for "Study Comparing the Efficacy of MEK162 Versus Dacarbazine in Unresectable or Metastatic NRAS Mutation-positive Melanoma" at ClinicalTrials.gov
- Hufford A (December 2015). "Array BioPharma Has Successful Trial for Cancer Drug Binimetinib". Wall Street Journal.
- "Array BioPharma announces Phase 3 binimetinib trial meets primary endpoint for NRAS-mutant melanoma". Metro Denver. December 2015. Archived from the original on 7 January 2021. Retrieved 14 March 2016.
- Array Bio submits marketing application in U.S. for lead product candidate in certain type of melanoma. June 2016
- House DW (1 September 2016). "FDA accepts Array Bio's NDA for binimetinib, action date June 30". Seeking Alpha.
- House DW (1 April 2016). "Array bags Phase 3 study of binimetinib in ovarian cancer; shares down 4%". Seeking Alpha.
- Adams B (20 March 2017). "Losing Nemo: Array pulls skin cancer NDA for binimetinib". Fierce Biotech.
External links
- "Binimetinib". Drug Information Portal. U.S. National Library of Medicine.