Transition metal carboxylate complex
Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.
-acetate-tetrahydrate-3D-balls.png.webp)
Acetate and related monocarboxylates
Structure and bonding
Carboxylates bind to single metals by one or both oxygen atoms, the respective notation being κ1- and κ2-. In terms of electron counting, κ1-carboxylates are "X"-type ligands, i.e., a pseudohalide-like. κ2-carboxylates are "L-X ligands", i.e. resembling the combination of a Lewis base (L) and a pseudohalide (X). Carboxylates are classified as hard ligands, in HSAB theory.
- Structures of Selected Metal Acetates
2.png.webp) Silver acetate
Silver acetate
4.svg.png.webp) Molybdenum(II) acetate, illustrating the Mo-Mo quadruple bond.[1]
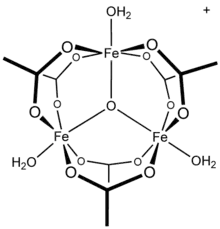
Molybdenum(II) acetate, illustrating the Mo-Mo quadruple bond.[1]![[CoO(acetate)]4, the "Das cubane"](../I/DasCubane.svg.png.webp) [CoO(acetate)]4, the "Das cubane"
[CoO(acetate)]4, the "Das cubane"
For simple carboxylates, the acetate complexes are illustrative. Most transition metal acetates are mixed ligand complexes. One common example is hydrated nickel acetate, Ni(O2CCH3)2(H2O)4, which features intramolecular hydrogen-bonding between the uncoordinated oxygens and the protons of aquo ligands. Stoichiometrically simple complexes are often multimetallic. One family are the basic metal acetates, of the stoichiometry [M3O(OAc)6(H2O)3]n+.[2]
2(H2O).svg.png.webp)
Homoleptic complexes
Homoleptic carboxylate complexes are usually coordination polymers. But exceptions exist.
- A molecular monocarboxylate is silver acetate, Ag2(OAc)2.
- Molecular diacetates are more common. Several diacetates adopt the Chinese lantern structure. Well studied examples include the dimetal tetraacetates (M2(OAc)4) including rhodium(II) acetate, copper(II) acetate, molybdenum(II) acetate, and chromium(II) acetate. Platinum diacetate and palladium diacetate feature Pt4 and Pd3 cores, further illustrating the tendency of acetate ligands to stabilize multimetallic structures.
- Mononuclear triacetates include derivatives of 1-adamantanecarboxylic acid, which have the formula [M(O2CC10H11)4]− (M = Co, Ni, Zn).[4]
Reactions and applications
Attempts to prepare some carboxylate complexes, especially for electrophilic metals, often gives oxo derivatives. Examples include the oxo-acetates of Fe(III), Mn(III), and Cr(III).
Metal acetates are common catalysts or precatalysts. Particularly useful are the ethylhexanoates and related metallic soaps.[5] These lipophilic complexes are used as catalysts in oxidation reactions, e.g. oil drying agents.
Synthesis
Many methods allow the synthesis of metal carboxylates. From preformed carboxylic acid, the following routes have been demonstrated:[6]
- acid-base reactions: LnMOR' + RCO2H → LnMO2CR + R'OH
- protonolysis: LnMalkyl + RCO2H → LnMO2CR + alkane
- oxidative addition: LnM + RCO2H → Ln(H)MO2CR
From preformed carboxylate, salt metathesis reactions are common:
- LnMCl + RCO2Na → LnMO2CR + NaCl
Metal carboxylates can be prepared by carbonation of highly basis metal alkyls:
- LnMR + CO2 → LnMO2CR
Reactions
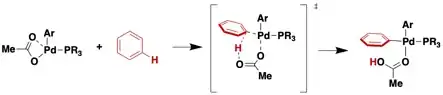
A common reaction of metal carboxylates is their displacement by more basic ligands. Acetate is a common leaving group. They are especially prone to protonolysis, which is widely used to introduce ligands, displacing the carboxylic acid. In this way octachlorodimolybdate is produced from dimolybdenum tetraacetate:
- Mo2(O2CCH3)4 + 8 HCl → [Mo2Cl8]2− + 4 CH3CO2H
Acetates of electrophilic metals are proposed to function as bases in concerted metalation deprotonation reactions.[7]
Pyrolysis of metal carboxylates affords acid anhydrides and the metal oxide. This reaction explains the formation of basic zinc acetate from anhydrous zinc diacetate.
In some cases, monodentate carboxylates undergo O-alkylation to give esters. Strong alkylating agents are required.
Di- and polycarboxylates
Benzenedi- and tricarboxylates
Metal organic frameworks, porous, three-dimensional coordination polymers, are often derived from metal carboxylate clusters. These clusters, called secondary bonding units (SBU's), are often linked by the conjugate bases of benzenedi- and tricarboxylic acids.[8]
Aminopolycarboxylates
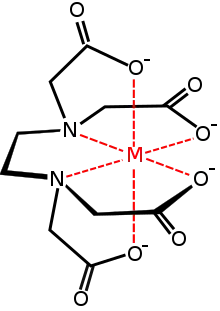 metal complex with the EDTA anion
metal complex with the EDTA anion2_anion.svg.png.webp) Ferric bis(iminodiacetate)
Ferric bis(iminodiacetate)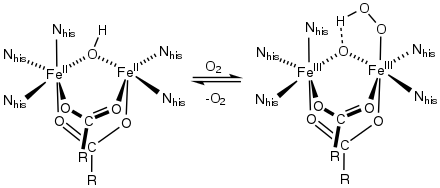 Active site of hemerythrin, an O2-carrying iron-carboxylate
Active site of hemerythrin, an O2-carrying iron-carboxylate
A commercially important family of metal carboxylates are derived from aminopolycarboxylates, e.g., EDTA4-. Related to these synthetic chelating agents are the amino acids, which form large families of amino acid complexes. Two amino acids, glutamate and aspartate, have carboxylate side chains, which function as ligands for iron in nonheme iron proteins, such as hemerythrin.[9]
Related topics
References
- Brignole, Alicia B.; Cotton, F. A. (1972). "Rhenium and Molybdenum Compounds Containing Quadruple Bonds". Inorganic Syntheses. 13: 81–89. doi:10.1002/9780470132449.ch15.
- Catterick, Janet; Thornton, Peter (1977). Structures and Physical Properties of Polynuclear Carboxylates. Advances in Inorganic Chemistry and Radiochemistry. Vol. 20. pp. 291–362. doi:10.1016/S0065-2792(08)60041-2. ISBN 9780120236206.
- Zhang, Gao; Lin, Jian; Guo, Dong-Wei; Yao, Shi-Yan; Tian, Yun-Qi (2010). "Infinite Coordination Polymers of One- and Two-dimensional Cobalt Acetates". Zeitschrift für Anorganische und Allgemeine Chemie. 636 (7): 1401–1404. doi:10.1002/zaac.200900457.
- Fursova, E. Yu.; Romanenko, G. V.; Tolstikov, S. E.; Ovcharenko, V. I. (2019). "Mononuclear Transition Metal Adamantane-1-Carboxylates". Russian Chemical Bulletin. 68 (9): 1669–1674. doi:10.1007/s11172-019-2610-4. S2CID 203592748.
- Nora, Angelo; Szczepanek, Alfred; Koenen, Gunther (2001). "Metallic Soaps". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a16_361. ISBN 3527306730.
- García-Rodríguez, Raúl; Hendricks, Mark P.; Cossairt, Brandi M.; Liu, Haitao; Owen, Jonathan S. (2013). "Conversion Reactions of Cadmium Chalcogenide Nanocrystal Precursors". Chemistry of Materials. 25 (8): 1233–1249. doi:10.1021/cm3035642.
- Ackermann, Lutz (2011-03-09). "Carboxylate-Assisted Transition-Metal-Catalyzed C−H Bond Functionalizations: Mechanism and Scope". Chemical Reviews. 111 (3): 1315–1345. doi:10.1021/cr100412j. ISSN 0009-2665. PMID 21391562.
- Tranchemontagne, David J.; Mendoza-Cortés, José L.; o'Keeffe, Michael; Yaghi, Omar M. (2009). "Secondary Building Units, Nets and Bonding in the Chemistry of Metal–Organic Frameworks". Chemical Society Reviews. 38 (5): 1257. doi:10.1039/b817735j. PMID 19384437.
- Jasniewski, Andrew J.; Que, Lawrence (2018). "Dioxygen Activation by Nonheme Diiron Enzymes: Diverse Dioxygen Adducts, High-Valent Intermediates, and Related Model Complexes". Chemical Reviews. 118 (5): 2554–2592. doi:10.1021/acs.chemrev.7b00457. PMC 5920527. PMID 29400961.