Methoxymethyl ether
In organic chemistry, a methoxymethyl ether is a functional group with the formula ROCH2OCH3, abbreviated MOM. Methoxymethyl ethers are often employed in organic synthesis to protect alcohols. They are usually derived from 2-methoxymethyl chloride.[1][2] Closely related to MOM ethers are methoxyethoxymethoxy (MEM) protecting groups, introduced using 2-methoxyethoxymethyl chloride. The MEM protecting groups are more easily installed and more easily removed.
Protection
Typically, the alcohol to be protected is deprotonated with a non-nucleophilic base such as N,N-diisopropylethylamine (DIPEA) in dichloromethane followed by addition of the chloromethyl reagent.[3]<
Although not relevant to protecting groups, MOM groups are installed by reaction of chloromethyl ethers with methoxide[4] and by the acid-catalyzed reaction of alcohols with dimethoxymethane.[5]
Deprotection
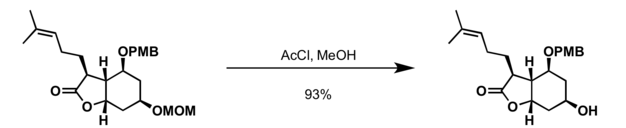
The MOM and the MEM protecting groups can be cleaved with a range of Lewis and Bronsted acids.[6]
Safety
Chloromethyl methyl ether and chloromethoxyethoxymethane, like other chloroalkyl ethers, are strong alkylating agents with attendant dangers. These compounds are human carcinogen.[7]
References
- Wuts, Peter G. M.; Greene, Theodora W. (2006). Greene's Protective Groups in Organic Synthesis, Fourth Edition - Wuts - Wiley Online Library. doi:10.1002/0470053488. ISBN 9780470053485. S2CID 83393227.
- Enders, Dieter; Geibel, Gunter; Osborne, Simon (2000-04-17). "Diastereo- and Enantioselective Total Synthesis of Stigmatellin A". Chemistry – A European Journal. 6 (8): 1302–1309. doi:10.1002/(SICI)1521-3765(20000417)6:8<1302::AID-CHEM1302>3.0.CO;2-J. PMID 10840951.
- Kobayashi, Yusuke; Matsuda, Atsushi; Ushimaru, Tomoya; Haradaournal=Organic Syntheses, Toshiro (2019). "Preparation of (R)-3-(3,5-Bistrifluoromethylphenyl)-1,1'-bi-2-naphthol". Organic Syntheses. 96: 312–332. doi:10.15227/orgsyn.096.0312.
- William F. Bailey, Matthew W. Carson, Lyn M. J. Zarcone (1998). "Selective Protection of 1,3-Diols at the More Hindered Hydroxy Group: 3-(Methoxymethoxy)-1-Butanol". Organic Syntheses. 75: 177. doi:10.15227/orgsyn.075.0177.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kohei Tamao, Yoshiki Nakagawa, and Yoshihiko Ito (1996). "Regio- AND Stereoselective Intramolecular Hydrosilylation of a-Hydroxy Enol Ethers: 2,3-syn-2-Methoxymethoxy-1,3-nonanediol". Organic Syntheses. 73: 94. doi:10.15227/orgsyn.073.0094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Amano, Seiji; Takemura, Noriaki; Ohtsuka, Masami; Ogawa, Seiichiro; Chida, Noritaka (1999-03-26). "Total synthesis of paniculide A from d-glucose". Tetrahedron. 55 (13): 3855–3870. doi:10.1016/S0040-4020(99)00096-4.
- Bis(chloromethyl) Ether and Technical-Grade Chloromethyl Methyl Ether CAS Nos. 542-88-1 and 107-30-2, Report on carcinogens, Eleventh edition